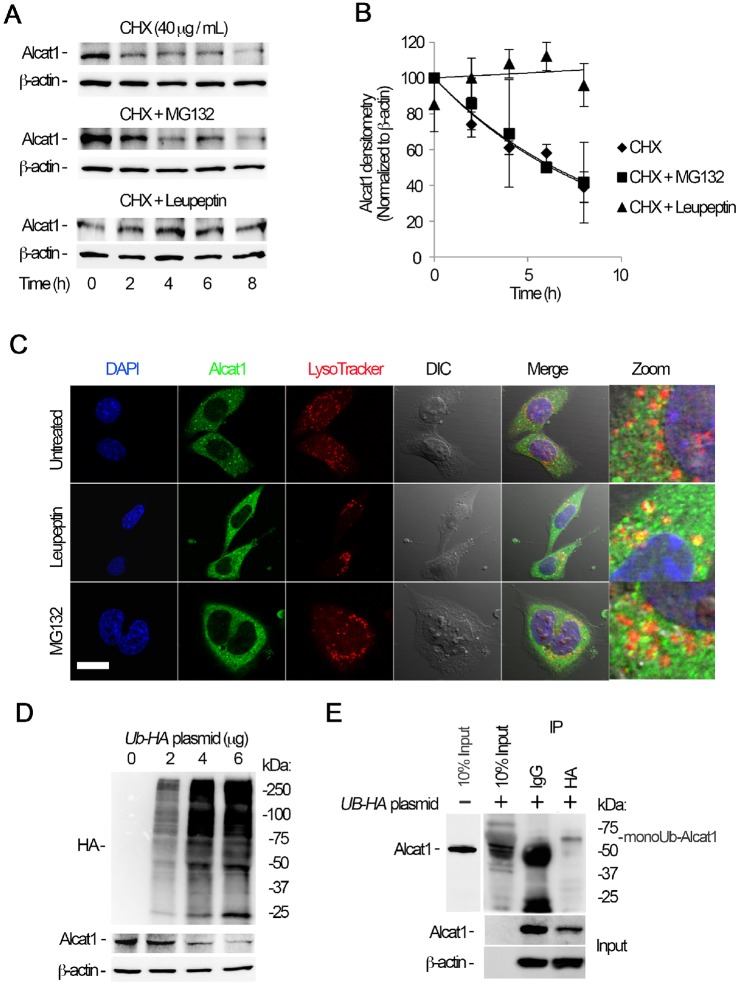
Fig. 4.
Alcat1 is monoubiquitylated and degraded through a lysosomal pathway. (A) MLE cells were treated with cycloheximide (CHX), CHX with MG132, or CHX with leupeptin, and cells were harvested at a variety of time points; lysates were analyzed by immunoblotting for Alcat1 and β-actin. (B) The bands shown in A were quantified, and the densitometry results are shown graphically. (C) Alcat1 colocalizes with lysosomes. Cells were treated with leupeptin or MG132 for 6 h and then immunostained for Alcat1 or with LysoTracker. The nucleus was visualized with DAPI staining. Untreated cells were used as a control. Scale bar: 10 μm. (D) Ectopically expressed HA–ubiquitin stimulates Alcat1 degradation. Various amounts of HA–ubiquitin (UB-HA) plasmids were introduced into MLE cells for 48 h. Cell lysates were processed for immunostaining of HA, Alcat1 and β-actin. (E) Cell expressing HA–ubiquitin were subjected to immunoprecipitation of HA, followed by immunoblotting for Alcat1 and β-actin. Data in each panel represent n=3 separate experiments. IP, immunoprecipitation; monoUb-Alact1, monoubiquitylated Alcat1. Means±s.e.m. are shown.