Fig. 4.
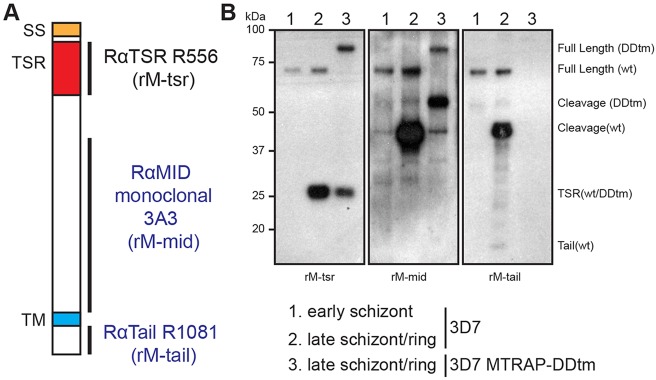
MTRAP organisation and profile of domain-specific antibodies. (A) MTRAP consists of a signal sequence (SS, orange), two thrombospondin repeat domains (TSR, red) and a single transmembrane domain (TM, blue), with a short C-terminal cytoplasmic tail. In order to track the various cleavage products of MTRAP we generated a rabbit monoclonal antibody (3A3) against the unstructured mid domain (rM-mid) and rabbit antiserum (R1081) against the short C-terminal tail (rM-tail). These antibodies complement the previously published MTRAP TSR (R556) antiserum (rM-tsr). (B) Analysis of 3D7 schizont and mixed schizont and ring (schizont/ring)-stage parasites along with schizont/ring-stage parasites expressing a DDtm-tagged version of MTRAP (MTRAPDDtm), were used to test antibody recognition. Immunoblot analysis labelled with rM-tsr antiserum identified full-length wild-type (wt) MTRAP at ∼75 kDa, full-length MTRAPDDtm at ∼90 kDa, and the cleaved MTRAP TSR domain at ∼27 kDa in both late schizont/ring samples as expected. rM-mid antibodies also recognised the full-length products, demonstrating its ability to specifically label MTRAP. Additionally, rM-mid recognised at least one product at ∼44 kDa in 3D7 samples. A corresponding band running at ∼55 kDa in MTRAPDDtm parasites confirms that this is a cleavage product of MTRAP, which maintains its C-terminal tail. In some samples a second cleavage product running at ∼38 kDa was also seen (data not shown). rM-tail antiserum recognised the same full-length and cleavage products in 3D7 samples. Additionally, a faint band was consistently labelled running at ∼15 kDa in samples involving early ring stage parasites, but was absent in schizont stage parasites. We believe this corresponds to the MTRAP C-terminal tail stub following intramembrane cleavage. rM-tail antiserum was unable to label C-terminally-tagged versions of MTRAP.