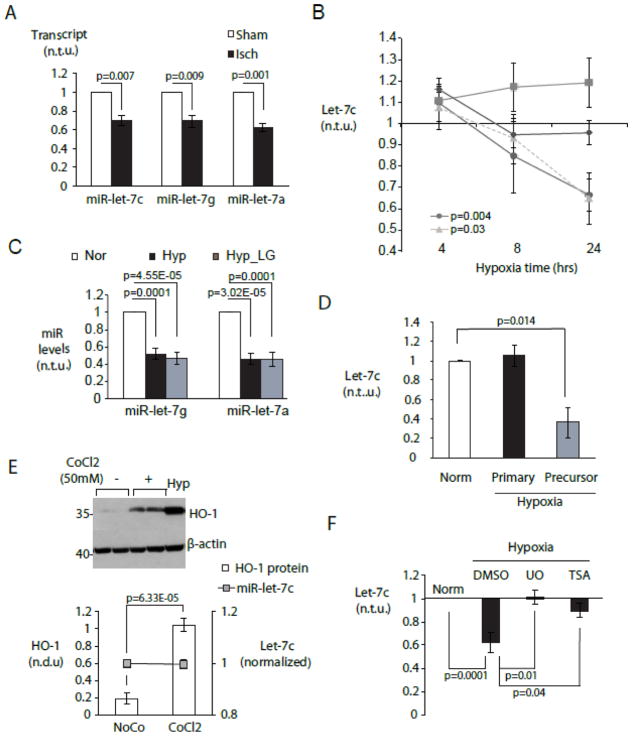
Figure 1. Cell type-specific repression of miR-let-7 by hypoxia in cardiac myocytes.
A. Acute myocardial ischemia in vivo represses multiple miR-let-7 species. MiR-let-7c,-7g and -7a were quantitated in mouse myocardium 2hr after LAD ligation (black bars) or a sham procedure (white bars) using a Taqman low-density microfluidic array (n=3). B. Myocyte-specific repression of miR-let-7c is mediated by hypoxia. Control neonatal rat ventricular myocytes were cultured under normoxia with 5 mM glucose. Experimental groups included normoxia without glucose (LG, diamonds), hypoxia with glucose (circles) and hypoxia without glucose (triangles). Cardiac fibroblasts (squares) were cultured under normoxia with glucose. MiR-let-7c was quantitated by RT-PCR at 4, 8 and 24 hours. C. Hypoxia represses miR-let-7g and -7a. CMs were cultured as in (B) and miRNAs were quantitated at 24 hour. For (B) and (C), graphs display miRNA expression levels in experimental conditions relative to normoxic cells (n=5). D. Hypoxia downregulates miR-let-7 at post-transcriptional level. Primary and precursor miR-let-7c transcripts were quantitated by qPCR in CM cultured as in (B) and expressed relative to those in normoxic CMs (n=6) E. Chemical induction of HIF1 is insufficient to downregulate let-7. Cardiac myocytes were subjected to treatment with 50mM cobalt chloride (CoCl2) and vehicle (NoCo), and expression of HO-1 protein and miR-let-7c were assayed by immunoblot and/or RT-PCR respectively. (top) Representative Western blot. (bottom) HO-1 protein/β-actin and miR-let-7c transcript levels (n=6). Hypoxia treated CM serves as positive control. F. Hypoxic downregulation of let-7 requires MAPK/ERK and class I-II histone deacetylase activity. MiR-let-7c levels under hypoxic NRVMs in the presence and absence of the non-selective HDAC inhibitor trichostatin A (TSA) (10nM) and the MEK1/2 inhibitor U0126 (U0, (10μM)) (n=4). Norm= normoxic culture.