Abstract
Hepatitis E virus (HEV), norovirus (NoV), and astrovirus (AstV) are enterically-transmitted viral pathogens causing epidemic or endemic hepatitis (HEV) and gastroenteritis (NoV and AstV) respectively in humans, leading to significant morbidity and mortality worldwide. While a recombinant subunit vaccine against HEVs is available in China, there is no commercial vaccine or antiviral against NoV or AstV. We report here our development of a trivalent vaccine against the three viral pathogens through our new polymer vaccine technology. All HEV, NoV, and AstV are non-enveloped RNA viruses covered by a protein capsid, featuring surface protruding (P) proteins that are responsible for virus–host interaction. These dimeric P protein selicit neutralizing antibody and are good targets for subunit vaccine development. The trivalent subunit vaccine was developed by fusion of the dimeric P domains of the three viruses together that formed tetramers. This trivalent vaccine elicited significantly higher antibody responses in mice against all three P domains than those induced by a mixture of the three free P domains (mixed vaccine). Furthermore, the post-immune antisera of the trivalent vaccine showed significantly higher neutralizing titers against HEV infection in cell culture and higher blocking activity on NoV binding to HBGA ligands than those of the post-immune antisera of the mixed vaccine. Thus, the trivalent vaccine is a promising vaccine candidate against HEV, NoV, and AstV.
Keywords: Oligomeric complex, Vaccine development, Trivalent vaccine, Vaccine platform, Norovirus, Hepatitis E virus, Astrovirus
1. Introduction
Noroviruses (NoVs) and astroviruses (AstVs), members of the families Caliciviridae and Astroviridae, respectively, are common causative agents of epidemic acute gastroenteritis in humans, leading to significant morbidity and mortality [1,2]. It is estimated that NoV alone causes approximately 21 million infections in the United States and claim over 200,000 deaths worldwide every year [1]. Although our knowledge on AstV epidemiology remains limited, seroprevalence studies carried out in the United States showed that 90% of children have antibody to human AstV-1 by the age of nine, indicating that AstV infection is common [2]. Both NoVs and AstVs are transmitted through fecal-oral route. Hepatitis E viruses (HEVs), members of the family Hepeviridae [3], are another common enterically-transmitted viral pathogen, causing non-A, non-B viral hepatitis, hepatitis E [4]. World Health Organization (WHO) estimates that there are 20 million HEV infections worldwide each year, leading to over 3 million acute cases of hepatitis E and 56,600 deaths [5]. Thus, NoVs, AstV, and HEVs constitute great burdens to public health. Unfortunately, except for a subunit vaccine against HEVs that is currently available only in China [6], there is no prophylactic or therapeutic approach against NoVs or AstVs.
In spite of differences in their genetic origins and classifications, NoVs, AstVs, and HEVs share important genetic, structural, and antigenic features. In fact, HEV was once classified in the same family Caliciviridae as NoVs and AstVs. They are nonenveloped RNA viruses with a single-stranded, positive-sense RNA genome in similar lengths (~7.5 kb), consisting of three open reading frames (ORF). All three viruses are encapsulated by a protein capsid in similar sizes (27–37 nm) that is made by a single major structural protein, the capsid protein [7–9]. The viral capsids are featured by an interior shell and many exterior protrusions that are made by the shell (S) and the protruding (P) domain of the capsid protein, respectively. While the interior shells serve as the basic scaffold of the icosahedral virions, the surface protruding P dimers are responsible for virus–host interactions [10–14], including viral attachment and penetration (reviewed in [15–19]). As a result, the P domains can elicit neutralizing antibodies against infection of the three viruses [20–22], respectively, and therefore are attractive targets for subunit vaccine development against the three viruses [20,23]. Like those on the viral capsid, heterologous expressions of the P domain proteins by Escherichia coli self-assemble into P domain dimers [10,13,24–26].
While there is no commercial vaccine or antiviral against NoVs and AstV, the first non-replicating subunit HEV vaccine that is based the recombinant P domain particles (HEV 239) has been available in China [6]. However, a commercial HEV vaccine remains lacking in other nations. Due to the lack of a cell culture system for NoVs, different subunit NoV vaccines are under development [27], among which VLP-based vaccine has been in phase II clinical trial [28,29]. Recently we have developed a simple technology to turn small antigens into large polymers for improved immunogenicity for vaccine development [30,31]. A bivalent vaccine against HEV and NoV was made and tested based on this approach [32]. In this study, we took further advantage of this technology to develop a trivalent vaccine against HEV, NoV, and AstV. Fusion protein complexes consisting of the dimeric P domains of the three viruses were designed, produced, and evaluated for its immune responses to all three viral antigens. Our results show that the fusion P protein complex is a promising trivalent vaccine candidate against the three enterically-transmitted viral pathogens.
2. Materials and methods
2.1. The trivalent P domain vaccine
The trivalent subunit vaccine, referred as AstV P-HEV P-NoV P, was a recombinant fusion protein that contains the P domain antigens of an avian AstV (GenBank AC#: NP 987088, residue 423–630)[26], an HEV of a zoonotic genotype 3 from a pig [33] (GenBank AC#: DQ079627, residue 452–617, a part of the E2 protein) [10,34], and a human NoV (GII.4, GenBank AC#: AY038600.3, residue 222–535) [12,24] (Fig. 1A). A cysteine-containing peptide (CDCRGDCFC) was added to the C-terminus of the HEV P domain to stabilize the protein [30]. A linker or hinge consisting of 12 glycines (12G) was added between two the P domains, while a hisx6 tag was added to the N-terminus of the fusion protein for purification purpose (Fig. 1A).
Fig. 1.
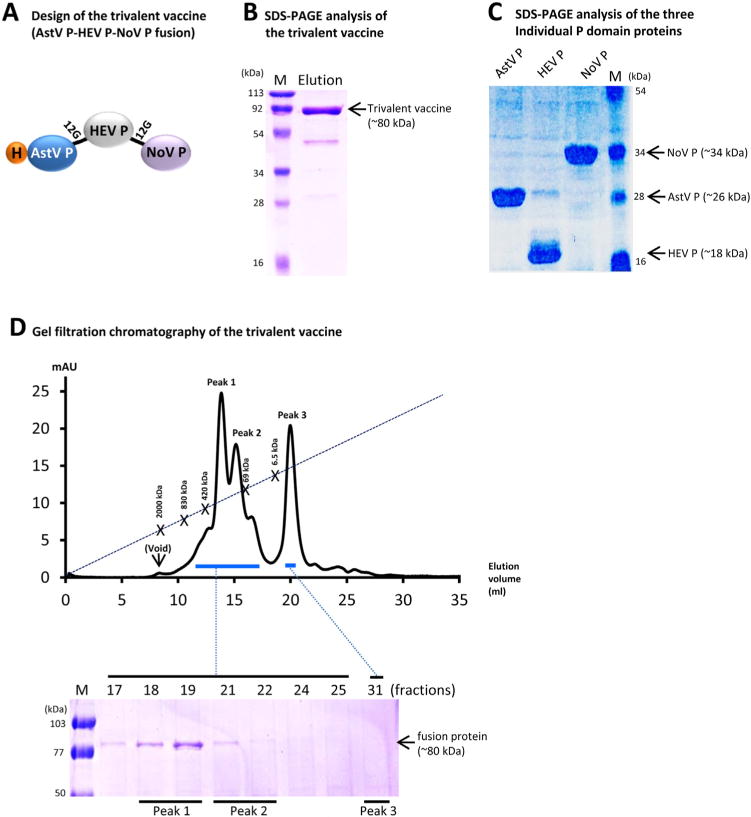
Design, production, and characterization of the trivalent vaccine. (A) Schematic diagram of the trivalent vaccine (AstV P-HEV P-NoV P) containing the P domain antigens of astrovirus (AstV), hepatitis E virus (HEV), and norovirus (NoV). The vaccine has two linkers consisting of 12 glycines (12G) between each two P domains and a histidinex6 tag (H) at the N-terminus. (B and C) SDS PAGE analysis of the affinity column-purified trivalent vaccine (~80 kDa) (B) and the individual P domain proteins of AstV (AstV P), HEV (HEV P), and NoV (NoV P) (C). (D) The elution curve of a gel filtration chromatography of the trivalent vaccine through the size-exclusion column Superdex 200 (10/300 GL, GE Healthcare Life Sciences). The major peaks of the gel-filtration were analyzed by SDS-PAGE that is shown below the elution curve. The three major peaks are indicated as Peak 1 (fractions 18/19), 2 (fractions 21/22), and 3 (faction 31), respectively. The elution ranges of the proteins are indicated by blue bars. The gel filtration columns were calibrated by the Gel Filtration Calibration Kit (GE Healthcare Life Sciences) and the purified recombinant P particle [36,46], small P particle [37], and P dimer [24] of norovirus (VA387). The elution positions of blue Dextran 2000 (~2000 kDa, void), P particle (~830 kDa), small P particle (~420 kDa), P dimer (~69 kDa), and aprotinin (~6.5 kDa) are indicated. Lane M represents pre-stained protein markers. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Plasmid constructs
The plasmid for expression of the trivalent vaccine was created based on the pQE30 vector (Qiagen, Germantown, MD), as well as the plasmids GST-NoV P and the GST HEV P constructs that were made previously [30]. The AstV P domain-encoding sequence was synthesized chemically through Genscript (Piscat-away, NJ). A plasmid containing pQE30 and AstV P-12G-HEV P fusion gene (designated as pQE30 and AstV P-12G-HEV P) was first constructed using two primer pairs, P2083/P2104 (AstV P) and P2062/P2105 (HEV P) (Table 1 in the Supplemental file). Another two primer pairs, P2083/P2142 and P2143/P2144 with BamHI/BsmBI and BsmBI/SalI sites, respectively (Table 1), were then used to amplify AstV P-12G-HEV P and NoV P-encoding cDNA sequences, respectively, followed by enzyme digestion, ligation and cloning into the pQE30 vector (Fig. 1A)[32]. The plasmid constructs for production of dimeric AstV P, HEV P and NoV P domains were made by cloning the corresponding coding sequences into the pQE30 vector, respectively.
Table 1.
Primers that were used to generate expression constructs of recombinant proteins.
| Construct | Name | Sequence (5′ to3′)a | Sense | Enzyme | Note |
|---|---|---|---|---|---|
| pQE30-AstV P-12G-HEV P | P2083 | GCACGGATCCTCTATCTACCTGCCGCTGC | + | BamHI | AstV P domain |
| P2104 | ATATCGTCTCCTCCGCCTCCGCCTCCGCCTCCGCCTCCGCCTCCGCCGAACTGAACGGTACG | − | BsmBI | AstV P domain (contains sequences for 12 Glycines) | |
|
| |||||
| P2062 | TATTCGTCTCCCGGATCTCCGGCTCCATCTCGTCCGTTCTCTGTTC | + | BsmBI | HEV P domain | |
| P2105 | GCACAAGCTTTTACGGGTAGTCAACGGTGTCTTC | − | HindIII | HEV P domain | |
|
| |||||
| pQE30-AstV P-12G-HEV P-12G-NoV P | P2142 | ATATCGTCTCCCGCCGCAAAAGCAATCGCCACGGCAATCGCACG | − | BsmBI | HEV P domain |
| P2143 | TATTCGTCTCCGGCGGAGGCGGAGGCGGAGGCGGAGGCGGAGGCGGATCAAGAACTAAACCATTC | + | BsmBI | NoV P domain (contains sequences for 12 Glycines) | |
| P2144 | ATATGTCGACTTACCCCGCTCCATTTCCCATGGGGGCAAGTGTGTAGAACTG | − | SalI | NoV P domain | |
enzyme sites are underlined
2.3. Production and purification of recombinant proteins
The recombinant proteins were expressed in E. coli (BL21, DE3) as described previously [24,30]. The hisx6-tagged trivalent vaccine and the dimeric P domain proteins were purified by TALON CellThru Resin (ClonTech) according to the instruction of the manufactory.
2.4. SDS-PAGE and protein quantitation
Purified proteins were analyzed by SDS-PAGE using 10% separating gels. Proteins were quantitated by SDS-PAGE using serially diluted bovine serum albumin (BSA, Bio-Rad) as standards on same gels [35].
2.5. Gel filtration chromatography
This was carried out to characterize the size of the trivalent vaccine as described elsewhere [24,30,36] through an AKTA Fast Performance Liquid Chromatography System (AKTA Pure 25L, GE Healthcare Life Sciences) via a size exclusion column (Superdex 200, 10/300 GL, GE Healthcare Life Sciences). The column was calibrated using gel filtration calibration kits (GE Healthcare Life Sciences) and purified NoV P particles (~830 kDa) [36], small P particles (~420 kDa) [37], and P dimers (~69 kDa) [24] as described previously. The protein identities in the peaks were further characterized by SDS-PAGE.
2.6. Immunization of mice
Female BALB/c mice (Harlan-Sprague-Dawley, Indianapolis, IN) at 3–4 weeks of age were divided into three basic groups (n = 6) for different immunizations. For vaccination without adjuvant, mice in the vaccination group were administered with the fused trivalent vaccine (15 μg/mouse) consisting of the three P domain antigens of AstV, HEV, and NoV for three times. Mice in the mixed vaccine group were immunized three times with same dose (15 μg/mouse) of the mixed vaccine that was a mixture of equal amount of the three individual free P domains of AstV (5 μg/mouse), HEV (5 μg/mouse), and NoV (5 μg/mouse). The third group was negative control mice that were administered with the same volume of phosphate buffered saline (PBS, pH7.4). Immunizations were performed intranasally without adjuvant. For vaccination with monophosphoryl lipid A (MPLA, 5 μg/dose) adjuvant, doses of both fused trivalent and the mixed vaccines were reduced to 7.5 μg/mouse. The mixed vaccine (7.5 μg/mouse) contained equal amount (2.5 μg/mouse) of each P domain of AstV, HEV, and NoV, respectively. Mice were immunized three times intranasally in 2-week intervals as described previously [30,35]. Blood was collected by retro-orbital capillary plexus puncture before each immunization and two weeks after the final immunization. Sera were processed from blood via a standard protocol.
2.7. Enzyme immunoassay (EIA)
EIA was carried out to measure the antibody titers of mouse antisera after immunization, as described previously [35]. Gel-filtration-purified P domain proteins of AstV, HEV, and NoV were used as antigens to determine the AstV-, HEV-, and NoV-specific antibodies, respectively. P domain antigens at 1 μg/ml were coated on 96-well microtiter plates and incubated with serially diluted mouse sera. Bound antibodies were detected by goat-anti-mouse secondary antibody-HRP conjugates (MP Biomedicals, Inc). Antibody titers were defined as the end-point dilutions with a cutoff signal intensity of OD450 = 0.15.
2.8. Histo-blood group antigen (HBGA) binding and blocking assays
The saliva-based HBGA binding assays that mimic NoV-host ligand attachment were performed as described elsewhere [38,39]. Briefly, diluted saliva samples with defined type A or B antigens were coated on 96-well microtiter plates and incubated with diluted the trivalent vaccine or NoV P domain at indicated concentration. The bound proteins were measured by guinea pig anti-NoV VLP antiserum, followed by an incubation of HRP-conjugated goat anti-guinea pig IgG (ICN Pharmaceuticals). The negative binding of HEV P domain protein to saliva was used as a negative control that was measured by HEV P specific antibody. The blocking assay that mimics neutralization of NoV-HBGA interaction by specific mouse antisera was basically a HBGA-P particle binding assay [36] with an extra step that NoV P particles (NoV surrogates) were pre-incubated with mouse sera for 1 h before the P particles were incubated with the coated type A saliva. The blocking rates were defined as reduction rates by comparing the optical density with and without blocking. The 50% blocking titer (BT50) was defined as the highest serum dilution causing a 50% reduction on the binding of NoV P particle to HBGAs.
2.9. HEV neutralization assay
The HEV neutralization by mouse sera was measured essentially as previously described [40,41] using the HEV Kernow P6 strain (genotype 3, kindly provided by Dr. S.U. Emerson, NIAID, NIH) and HepG2/C3A cells. Infectious titers of HEV expressed as focus forming units (FFU) were determined by a fluorescent-focus assay (FFA). ~50,000 HepG2/C3A cells/well were seeded in 96-well plates. The viruses (100 FFU/well) were mixed with the 2-fold serially diluted mouse sera for 2 h at 37 °C and then added to the cells. After a 2-h incubation, the inocula were discarded and replaced with maintenance medium. After further incubation for 5 days, the infected cells were fixed with 80% acetone and incubated with a rabbit anti-HEV ORF2 antibody, washed with PBST (1×PBS with 0.2% tween-20), and then incubated with Alexa Fluor® 488 conjugated Goat Anti-Rabbit IgG Antibody. The stained cells were visualized via a fluorescence microscope. The neutralization titers of the sera were defined as the highest serum dilution that can reduce at least 60% of infected cells compared to no serum controls.
2.10. Statistical analysis
Statistical differences among data sets were calculated by softwares GraphPad Prism 6 (GraphPad Software, Inc) using an unpaired, non-parametric t test. P-values were set at 0.05 (P < 0.05) for significant difference, and 0.01 (P < 0.01) for highly significant difference.
2.11. Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (23a) of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cincinnati Children’s Hospital Research Foundation (Animal Welfare Assurance no. A3108-01).
3. Results
3.1. Production of the trivalent vaccine
Previous study showed that fusion of two or three dimeric proteins together leads to the formation of polymers with significantly improved immunogenicity [30]. Based on this concept, three dimeric P domain antigens of AstV [26], HEV [10], and NoV [12] were fused together (Fig. 1A, see Section 2) as a trivalent vaccine. The fused vaccine was expressed in E. coli as a soluble protein (~80 kDa) at a yield of ~10 mg/l of bacterial culture (Fig. 1B). Gel filtration chromatography (Fig. 1D) revealed that the vast majority of the vaccine formed most likely tetramers with a molecular weight (MW) of ~320 kDa (peak 1, fractions 18 and 19), while some vaccine may formed dimers with a MW of ~160 kDa (peak 2, fractions 21 and 22). Peak 3 (fraction 31) did not reveal any visible protein signal on the SDS gel, most likely containing only the degraded small peptides. Individual P domain proteins of the three viruses were also made (Fig. 1C), which have been previously shown to form dimers, respectively [10,12,26].
3.2. The trivalent vaccine bound HBGAs
To examine whether the P domain antigens retain their native antigenic structures, we performed HBGA-binding assays using the trivalent vaccine and the free NoV P dimers. As expected, both trivalent vaccine and the free NoV P dimers exhibited binding activity to HBGAs (Fig. 2), indicating that the NoV P domain of the trivalent vaccine retains the native antigenic structures that are necessary for an effective vaccine candidate.
Fig. 2.
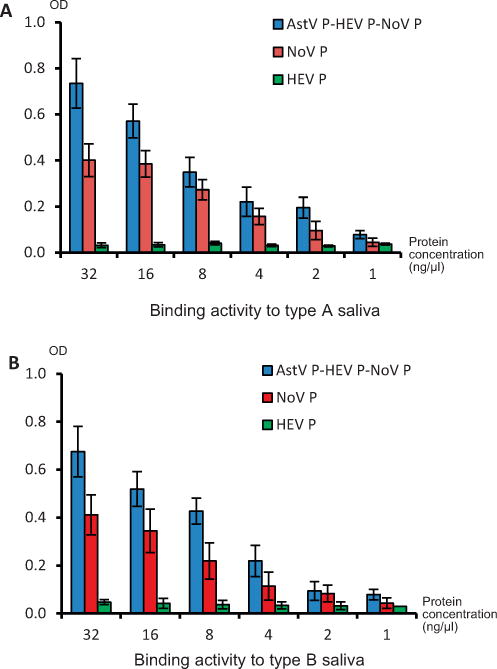
The trivalent vaccine retained histo-blood group antigen (HBGA) binding function. Human saliva samples with defined type A (A) or B (B) HBGA, respectively, were coated on microtiter plates. The trivalent vaccine (AstV P-HEV P-NoV P) and the norovirus P domain protein (NoV P) at different concentrations (X-axis) were incubated with the coated saliva and the bound NoV P proteins were detected by NoV-specific antisera. P domain of hepatitis E virus (HEP P) was used as negative control. The signal intensities of binding (OD) are shown in Y-axis.
3.3. The three P domains of the trivalent vaccine elicited IgG responses differently
We first administered the purified trivalent vaccine to mice intranasally without adjuvant. A mixed P domain vaccine containing equal amount of the three free P domains, designated as the mixed vaccine, was used as control. The resulting IgG titers showed that the three P domain antigens elicited different immune responses (Fig. 3). Both the trivalent and the mixed vaccines elicited the highest IgG titers to the NoV P domains (NoV P), followed by those to the AstV P domains (AstV P); while the HEV P domains (HEV P) induced the lowest IgG titers (Fig. 3A and B). When the vaccines were administered with MPLA adjuvant, the same order of IgG responses were observed (see Fig. 3C and D), but the IgG titers became higher and their differences among the P domain-specific IgG titers become smaller compared with those elicited by the vaccine without adjuvant (Fig. 3, compare A/B with C/D). This different immune responses may be due to the size differences among the three P domain antigens (NoV P > AstV P > HEV P, Fig. 1C) (see Section 4).
Fig. 3.
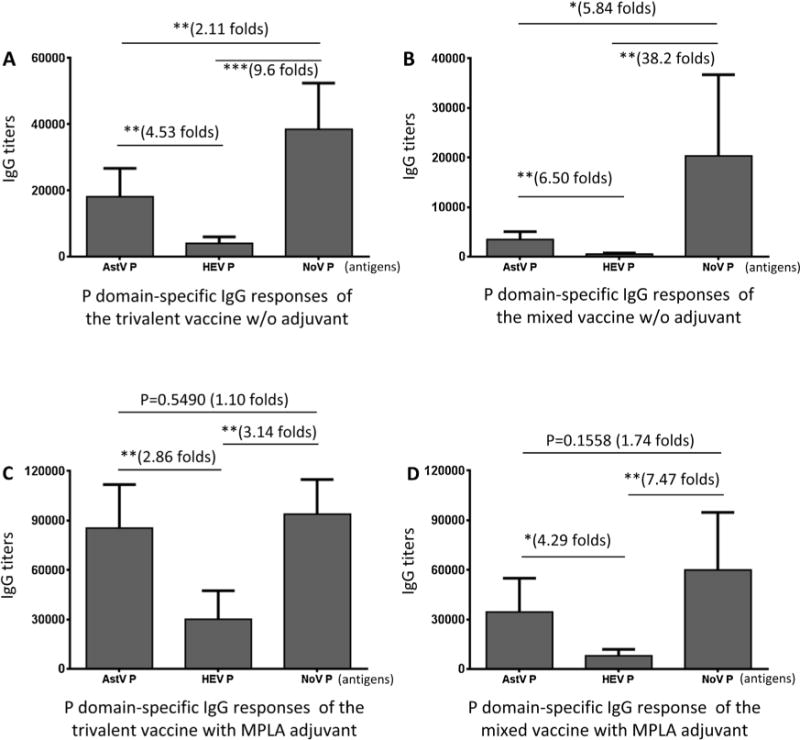
The three P domains of the trivalent vaccine elicited different IgG responses. Mice (n = 6) were immunized with equal amount of the trivalent (A and C) and mixed (B and D) vaccines intranasally without (A and B) or with (C and D) MPLA adjuvant. The IgG titers specific to individual P domain antigens of astrovirus (AstV P), hepatitis E virus (HEV P), and norovirus (NoV P) after immunizations were determined and compared. (A and B) Comparisons of the IgG titers after immunization of the trivalent (A) or mixed (B) vaccine without adjuvant. (C and D) Comparisons of the IgG titers after immunization of the trivalent (C) or mixed (D) vaccine with MPLA adjuvant. The differences between the data groups are indicated by folds, while their statistical differences are shown by star symbols (*P < 0.05, **P < 0.01, ***P < 0.001).
3.4. The trivalent vaccine increased IgG responses of the P domain components
Comparison of the IgG titers after immunization with the trivalent vaccine without adjuvant with those after immunization with the mixed vaccine showed that the trivalent vaccine elicited significantly higher IgG titers specific to the P domain antigens of AstV and HEV than those elicited by the mixed vaccine by 5.23 and 7.50 folds, respectively (Fig. 4A and B, Ps < 0.01). It was noted that, although the trivalent vaccine induced higher IgG titer against NoV P domain antigen than that induced by the mixed vaccine by 1.89 folds, their difference was not statistically significant (Fig. 4C, P = 0.0666). This may be due to the already high immune response to the relative large NoV P domain dimer (68 kDa) as explained above.
Fig. 4.
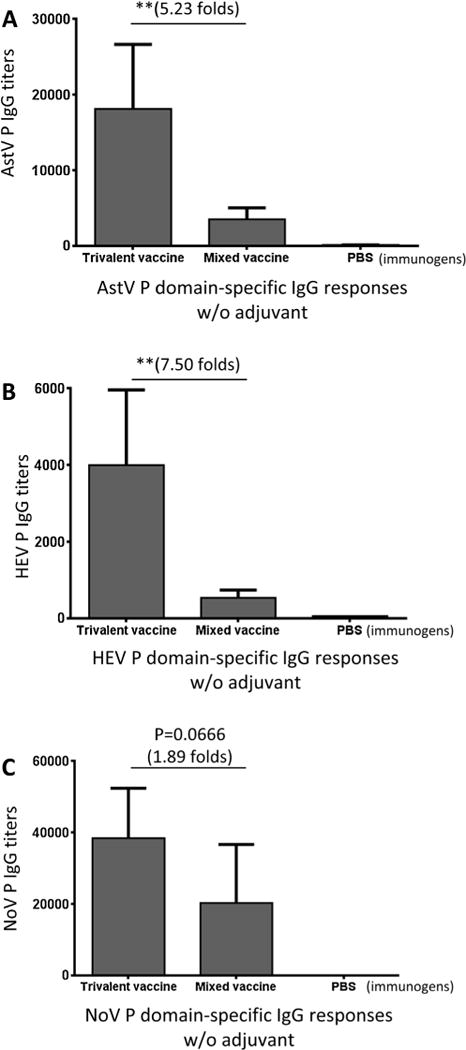
The trivalent vaccine increased IgG responses of P domain components. After immunization of mice (n = 6) with the trivalent and the mixed vaccines without adjuvant, the IgG responses specific to the P domain antigen of astrovirus (AstV), hepatitis E virus (HEV), and norovirus (NoV), respectively, were determined and compared. Phosphate buffered saline (PBS) was administered to mice as negative control. The resulting IgG titers specific to the P domain antigen of AstV (A), HEV (B), and NoV (C) were shown. The differences of the IgG titers between the two vaccines are indicated in folds, while their statistical differences are shown by star symbols (** P < 0.01).
When the vaccines were administered with MPLA adjuvant, the resulting IgG titers to all three P domain antigens were significantly higher (see below), but the differences between the two vaccines were smaller. Compared with the mixed vaccine, the trivalent vaccine increased the IgG titers against the P domains of AstV, HEV, and NoV by 2.46 (P < 0.01), 3.73 (Ps < 0.05), and 1.58 (P = 0.0673) folds, respectively (Fig. 5).
Fig. 5.
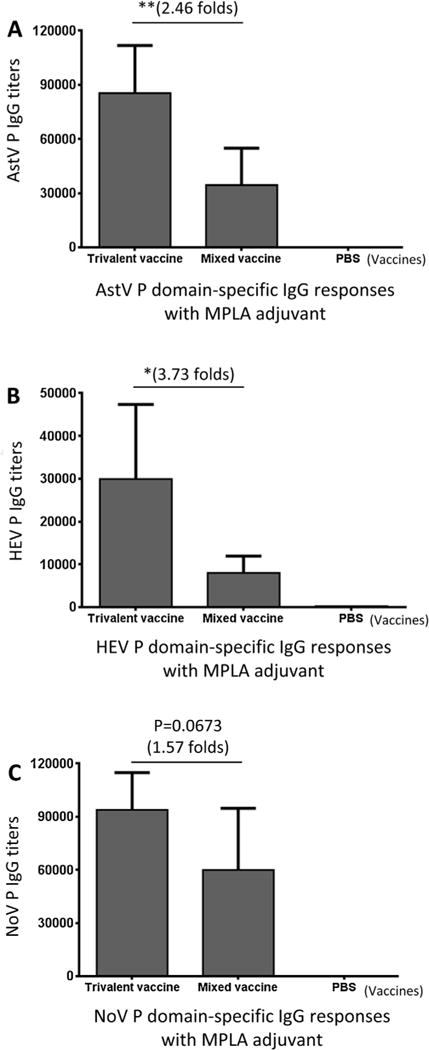
MPLA adjuvant increased the IgG responses of both trivalent and mixed vaccines, but reduced their differences. Mice (n = 6) were immunized with the two vaccines with MPLA adjuvant, respectively, the IgG titers specific to the P domain antigens of astrovirus (AstV), hepatitis E virus (HEV), and norovirus (NoV), respectively, were determined and compared. Phosphate buffered saline (PBS) was administered to mice as negative control. The resulting IgG titers specific to the P domain antigen of AstV (A), HEV (B), and NoV (C) were shown. The differences of the IgG titers between the two vaccines are indicated in folds, while their statistical differences are shown by star symbols (* P < 0.05, ** P < 0.01).
3.5. MPLA adjuvant increased the IgG responses of the P domains differently
As expected, MPLA adjuvant increased the IgG responses to all P domains of both trivalent and mixed vaccines (Fig. 6, Ps < 0.05). It was noted that, however, the increase magnitudes by the MPLA adjuvant to mixed vaccine were higher than those to the trivalent vaccine (Fig. 6, compared the increase folds of A with those of B). The antigen with a lower original immunogenicity appeared to gain a higher increase magnitude of IgG titer than the antigen with a higher original immunogenicity. For example, HEV P domain with the lowest IgG responses without an adjuvant gained 15.01 and 7.47 folds increase in the mixed and the trivalent vaccine, respectively, through the MPLA adjuvant. In contrast, NoV P domain with the highest IgG titers without an adjuvant showed less than three folds increase through the MPLA adjuvant. However, the P domain antigens with high immunogenicity without adjuvant remained highly immunogenic through the MPLA adjuvant.
Fig. 6.
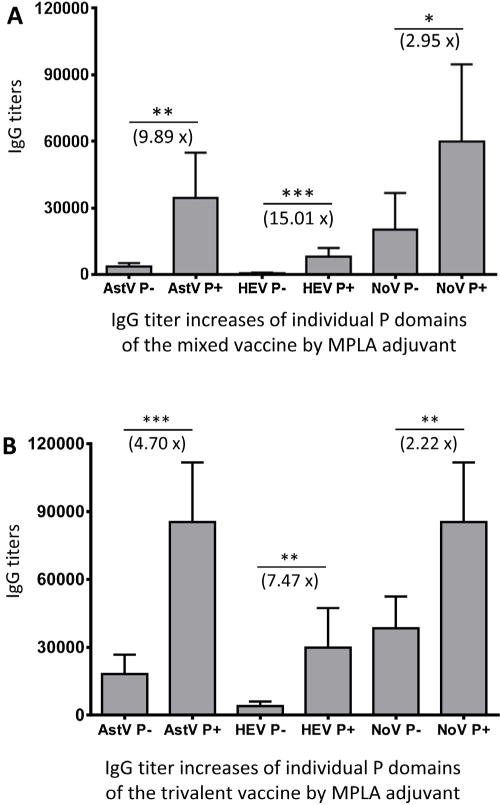
MPLA adjuvant increased the IgG responses to the three P domain antigens of the trivalent and the mixed vaccine differently. Mice were immunized with the mixed (A) and the trivalent (B) vaccines without (−) or with (+) MPLA adjuvant, the resulting IgG titers specific to individual P domain antigens of astrovirus (AstV P), hepatitis E virus (HEV P), and norovirus (NoV P) were determined and compared. The increased IgG titers (folds) by MPLA adjuvant are indicated, while their statistical differences are shown by star symbols (* P < 0.05, ** P < 0.01, *** P < 0.001).
3.6. The trivalent vaccine increased neutralizing titers against HEV replication
The mouse antisera after immunization of the two vaccines respectively were examined for their neutralization titers against HEV (strain Kernow P6) replication in HepG2/C3A cells. The neutralizing titers of mouse sera after immunization with the trivalent vaccine without adjuvant were higher than those of sera after immunization with the mixed vaccine by 1.67 folds (Fig. 7A, P = 0.1629). When vaccines were administered with the MPLA adjuvant, the trivalent vaccine elicited neutralizing titers were 4.01 folds higher than those elicited by the mixed vaccine (Fig. 7B, P < 0.05). These observations were supported by the fact that the MPLA adjuvant increased the neutralization titers of the trivalent vaccine by 2.17 folds (P = 0.0899), but not that of the mixed vaccine (Fig. 7C), probably due to the very low immunogenicity of the small HEV P domain (Figs. 1C and Fig. 6).
Fig. 7.
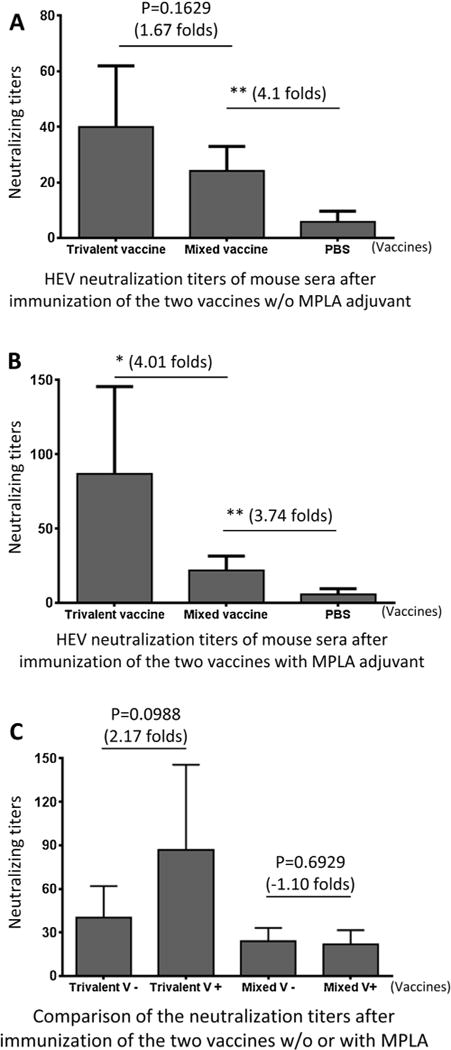
The post-immune antisera of the trivalent vaccine neutralized infection of hepatitis E virus (HEV). (A and B) Neutralization titers against HEV (Kernow P6) infection in HepG2/C3A cells were determined using the post-immune antisera after immunization of the trivalent and mixed vaccines, respectively, without (A) or with (B) MPLA adjuvant. (C) The neutralization titers of the mouse post-immune antisera of the trivalent and the mixed vaccines without (−) or with (+) MPLA adjuvant were compared. The differences of the neutralization titers between the two vaccines are indicated in folds, while their statistical differences are shown by star symbols (* P < 0.05, ** P < 0.01).
3.7. The trivalent vaccine increased blocking titers against NoV-host ligand attachment
Due to the lack of an effective cell culture system [42], a blocking assay of NoV-HBGA interaction has been developed as a surrogate NoV neutralization assay [29,43]. The mouse antis-era after immunization of the two vaccines were determined for their 50% blocking titers (BT50) against NoV P particle-type A-HBGA attachment [30,32]. The results showed that the trivalent vaccine-induced mouse antisera exhibited significantly higher BT50s than those of the antisera after immunization with the mixed vaccine (Fig. 8A and B, Ps < 0.05).
Fig. 8.
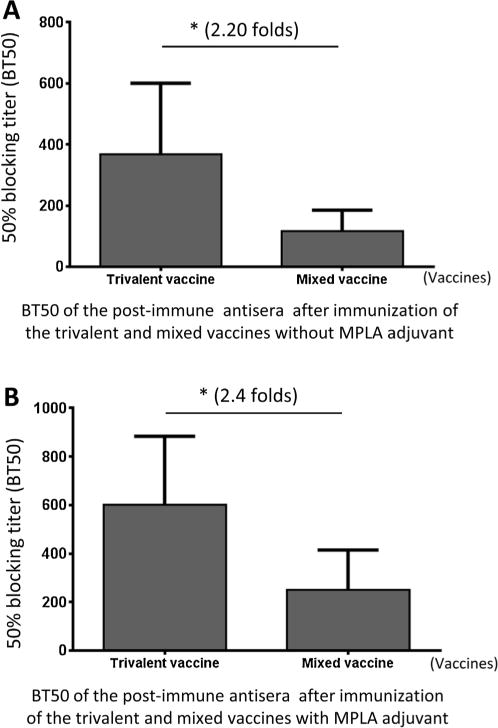
The post-immune antisera of the trivalent vaccine blocked NoV-host ligand attachment. The post-immune mouse antisera after immunization of trivalent vaccines without (A) and with (B) MPLA adjuvant exhibited significantly higher 50% blocking titers (BT50s) against NoV P particle-type A saliva attachment than those of the post-immune antisera after immunization with the mixed vaccine. The differences of the BT50s between the two vaccine are indicated in folds, while their statistical differences are shown by star symbols (* P < 0.05).
4. Discussion
We showed previously that fusion of two or three dimeric proteins together leads to formation of large polymers with significantly improved immunogenicity of the antigen components [30]. Based on this concept we designed and evaluated in this study a trivalent vaccine consisting of three dimeric viral neutralizing antigens, the P domains of AstV, HEV, and NoV, respectively. The three viral antigens were fused together through recombinant DNA technology with a flexible linker or hinge between each two P domain antigens. Although the resulting fusion protein, the trivalent vaccine, did not form very large polymers as expected, it did form most likely tetramers with molecular weights of ~320 kDa. These molecular sizes and valence are much larger than those of the individual free P domain antigens that are ~18 (HEV P domain), ~26 (AstV P domain), and ~34 (NoV P domain) kDa, respectively (Fig. 1). Based our previous studies [30–32,35], we hypothesized that this large, tetrameric trivalent vaccine would be able to elicit significantly improved immune responses to the three viral P domain antigens than those by a mixture of the three individual free P domain proteins (mixed vaccine). In this study, we provided evidence based on our data of preclinical animal trials to support this hypothesis. This included the significantly higher IgG responses to all three P domain antigens elicited by the trivalent vaccine than those induced by the mixed vaccine, as well as the significantly higher neutralization titers against HEVs and NoVs by the antisera after immunization with the trivalent vaccine compared with those after immunization of the mixed vaccine. Therefore, this fusion protein-based trivalent vaccine is a promising vaccine candidate against the enterically transmitted AstV, HEV, and NoV.
It remains unclear how the tetramers of this fusion protein was formed. All three P domains are dimeric and formed dimers in solution, when they were produced individually [10,12,26]. However, the fact that the fusion protein formed tetramers and dimers, but not polymers indicated that one or two of these three P domains might not actually form dimers in the fusion protein, probably due to certain structural interference as a result of the fusion of these three P domains together. The linkers or hinges consisting of 12 glycines between each two P domains was designed to provide flexibility among the P domains to prevent such structural interference, but this was clearly not good enough to avoid such issues. We showed that trivalent vaccine retained binding function to HBGAs (Fig. 2), the ligands of the NoV P domain [13,24,36,44], and the HBGA binding site is known to be bivalent that is formed by both protomers of the P dimer [13,44]. Thus, the NoV P domain must form dimer to bind the HBGA ligands. Since similar oligomers, instead of polymers were also observed in our previous study in developing a bivalent vaccine against NoV and HEV by fusion of the P domains of NoV and HEV together [32], future study is necessary to understand factors that interfere the dimerization of individual P domains for increased valency of these bi- and trivalent vaccines.
Another unexpected phenomenon of this trivalent vaccine was the differences in immunogenicity among three P domain antigens. The structural feature of the fusion protein-based trivalent vaccine ensured the molecular ratios among the three P domain antigens were equal. However, the immune responses to the three P domain antigens were significantly different (Fig. 3) with the highest IgG titers to the NoV P domain and the lowest titers to the HEV P domain. Similar scenarios occurred to both the trivalent and the mixed vaccines and their correlation with the sizes of the P domain antigens suggested that the sizes of the P domain antigens may be a major determinant of these different immune responses. The larger NoV P domain (34 kDa) may contain more antigenic epitopes than the smaller HEV P domain (18 kDa), resulting in higher and lower immune responses to the NoV and the HEV P domains, respectively.
Based on the same principle, a further factor that may explain the observed differences in the immune responses of the individual P domain antigens may be their dimerization. As discussed above, while not all three P domains of the trivalent vaccine dimerized, the NoV P domain formed most likely dimer as shown by its capability of binding to HBGA ligands. The dimerization of the NoV P domain further enlarged its molecular size or valency, leading to higher immune response. Vice versa, the relative low interaction of the HEV P domain [10] may make it a monomer in the trivalent vaccine, further explaining the observed low immunogenicity of the HEV P domain.
Our data showed that the MPLA (monophosphoryl lipid A) was an effective adjuvant to improve the immune responses of the trivalent vaccine. It significantly increased the IgG responses to all three viral antigens, resulting in higher neutralizing titers against HEVs and NoVs. The application of the MPLA adjuvant also led to more even immune responses to all three viral antigens (Fig. 3C). Since the MPLA, a detoxified form of the endotoxin lipopolysaccharide, is a new generation of Toll-like receptor agonists to be used as vaccine adjuvants in human populations [28,45], it should be associated with further development of this trivalent vaccine.
Acknowledgments
The research described in this article was supported by the National Institutes of Health, the National Institute of Allergy and Infectious Diseases (5R01 AI089634-01 to X.J. and R21 AI092434-01A1 to M.T.) and an institutional Innovation Fund of Cincinnati Children’s Hospital Medical Center to M.T.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.12.068.
References
- 1.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–31. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass RI, Noel J, Mitchell D, Herrmann JE, Blacklow NR, Pickering LK, et al. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch Virol Suppl. 1996;12:287–300. doi: 10.1007/978-3-7091-6553-9_31. [DOI] [PubMed] [Google Scholar]
- 3.Panda SK, Thakral D, Rehman S. Hepatitis E virus. Rev Med Virol. 2007;17:151–80. doi: 10.1002/rmv.522. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Subhadra S, Singh B, Panda BK. Hepatitis E virus: the current scenario. Int J Infect Dis: IJID. 2013;17:e228–33. doi: 10.1016/j.ijid.2012.11.026. official publication of the International Society for Infectious Diseases. [DOI] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proffitt A. First HEV vaccine approved. Nat Biotechnol. 2012;30:300. [Google Scholar]
- 7.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–90. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 8.Xing L, Li TC, Mayazaki N, Simon MN, Wall JS, Moore M, et al. Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J Biol Chem. 2010;285:33175–83. doi: 10.1074/jbc.M110.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishna NK, Cunnion KM. Human astrovirus coat protein: a novel C1 inhibitor. Adv Exp Med Biol. 2008;632:237–51. [PubMed] [Google Scholar]
- 10.Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, et al. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus–host interaction. PLoS Pathog. 2009;5:e1000537. doi: 10.1371/journal.ppat.1000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bu W, Mamedova A, Tan M, Xia M, Jiang X, Hegde RS. Structural basis for the receptor binding specificity of Norwalk virus. J Virol. 2008;82:5340–7. doi: 10.1128/JVI.00135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao S, Lou Z, Tan M, Chen Y, Liu Y, Zhang Z, et al. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol. 2007;81:5949–57. doi: 10.1128/JVI.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Tan M, Xia M, Hao N, Zhang XC, Huang P, et al. Crystallography of a lewis-binding norovirus, elucidation of strain-specificity to the polymorphic human histo-blood group antigens. PLoS Pathog. 2011;7:e1002152. doi: 10.1371/journal.ppat.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JM, Hutson AM, Estes MK, Prasad BV. Atomic resolution structural characterization of recognition of histo-blood group antigens by Norwalk virus. Proc Natl Acad Sci USA. 2008;105:9175–80. doi: 10.1073/pnas.0803275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–93. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Tan M, Jiang X. Norovirus–host interaction: implications for disease control and prevention. Expert Rev Mol Med. 2007;9:1–22. doi: 10.1017/S1462399407000348. [DOI] [PubMed] [Google Scholar]
- 17.Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan M, Jiang X. Norovirus–host interaction: multi-selections by human histo-blood group antigens. Trends Microbiol. 2011;19:382–8. doi: 10.1016/j.tim.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S, Miao J, Zheng Z, Wu T, Xie M, Tang M, et al. Putative receptor-binding sites of hepatitis E virus. J Gen Virol. 2008;89:245–9. doi: 10.1099/vir.0.83308-0. [DOI] [PubMed] [Google Scholar]
- 20.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine. 2005;23:2893–901. doi: 10.1016/j.vaccine.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Gu Y, Ge SX, Li SW, He ZQ, Huang GY, et al. Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine. 2005;23:2881–92. doi: 10.1016/j.vaccine.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 22.Fang H, Tan M, Xia M, Wang L, Jiang X. Norovirus P particle efficiently elicits innate, humoral and cellular immunity. PLoS ONE. 2013;8:e63269. doi: 10.1371/journal.pone.0063269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan M, Jiang X. Norovirus P particle: a subviral nanoparticle for vaccine development against norovirus, rotavirus and influenza virus. Nanomedicine (Lond) 2012;7:889–97. doi: 10.2217/nnm.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan M, Hegde RS, Jiang X. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J Virol. 2004;78:6233–42. doi: 10.1128/JVI.78.12.6233-6242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong J, Dong L, Mendez E, Tao Y. Crystal structure of the human astrovirus capsid spike. Proc Natl Acad Sci USA. 2011;108:12681–6. doi: 10.1073/pnas.1104834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuBois RM, Freiden P, Marvin S, Reddivari M, Heath RJ, White SW, et al. Crystal structure of the avian astrovirus capsid spike. J Virol. 2013;87:7853–63. doi: 10.1128/JVI.03139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan M, Jiang X. Vaccine against norovirus. Hum Vaccines Immunother. 2014;10 doi: 10.4161/hv.28626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atmar RL, Bernstein DI, Lyon GM, Treanor JJ, Al-Ibrahim MS, Graham DY, et al. Serological correlates of protection against a GII.4 norovirus. Clin Vaccine Immunol: CVI. 2015 doi: 10.1128/CVI.00196-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Huang P, Fang H, Xia M, Zhong W, McNeal MM, et al. Polyvalent complexes for vaccine development. Biomaterials. 2013;34:4480–92. doi: 10.1016/j.biomaterials.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Xia M, Huang P, Fang H, Cao D, Meng XJ, et al. Branched-linear and agglomerate protein polymers as vaccine platforms. Biomaterials. 2014;35:8427–38. doi: 10.1016/j.biomaterials.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Cao D, Wei C, Meng XJ, Jiang X, Tan M. A dual vaccine candidate against norovirus and hepatitis E virus. Vaccine. 2014;32:445–52. doi: 10.1016/j.vaccine.2013.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori Y, Matsuura Y. Structure of hepatitis E viral particle. Virus Res. 2011;161:59–64. doi: 10.1016/j.virusres.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad I, Holla RP, Jameel S. Molecular virology of hepatitis E virus. Virus Res. 161:47–58. doi: 10.1016/j.virusres.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan M, Huang P, Xia M, Fang PA, Zhong W, McNeal M, et al. Norovirus P particle, a novel platform for vaccine development and antibody production. J Virol. 2011;85:753–64. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan M, Jiang X. The p domain of norovirus capsid protein forms a sub-viral particle that binds to histo-blood group antigen receptors. J Virol. 2005;79:14017–30. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan M, Fang PA, Xia M, Chachiyo T, Jiang W, Jiang X. Terminal modifications of norovirus P domain resulted in a new type of subviral particles, the small P particles. Virology. 2011;410:345–52. doi: 10.1016/j.virol.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, Morrow AL, et al. Noroviruses bind to human ABO, lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 39.Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, et al. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol. 2005;79:6714–22. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanford BJ, Emerson SU, Purcell RH, Engle RE, Dryman BA, Cecere TE, et al. Serological evidence for a hepatitis E virus-related agent in goats in the United States. Transboundary Emerg Dis. 2012 doi: 10.1111/tbed.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emerson SU, Clemente-Casares P, Moiduddin N, Arankalle VA, Torian U, Purcell RH. Putative neutralization epitopes and broad cross-genotype neutralization of Hepatitis E virus confirmed by a quantitative cell-culture assay. J Gen Virol. 2006;87:697–704. doi: 10.1099/vir.0.81545-0. [DOI] [PubMed] [Google Scholar]
- 42.Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MP, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- 43.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–8. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Chen Y, Jiang X, Xia M, Yang Y, Tan M, et al. A unique human norovirus lineage with a distinct HBGA binding interface. PLoS Pathog. 2015;11:e1005025. doi: 10.1371/journal.ppat.1005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci: CMLS. 2008;65:3231–40. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan M, Fang P, Chachiyo T, Xia M, Huang P, Fang Z, et al. Noroviral P particle: structure, function and applications in virus–host interaction. Virology. 2008;382:115–23. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


