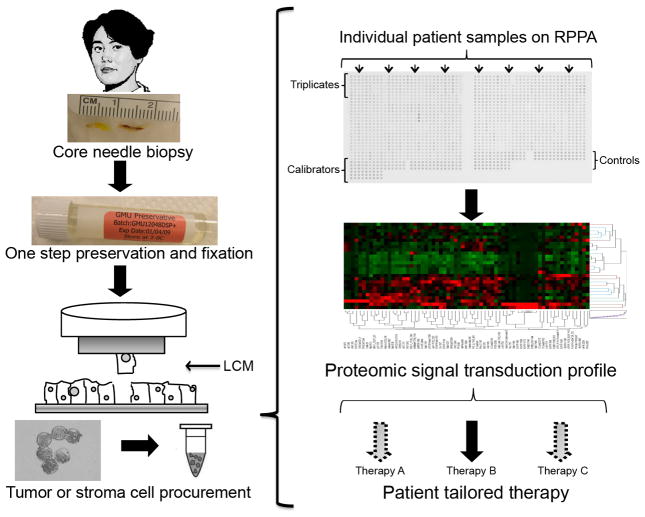
Fig. 1.
Reverse phase protein microarray (RPPA) workflow for clinical samples. Biopsy or surgical samples are immediately stabilized following procurement. Enriched cell populations are procured by Laser Capture Microdissection, followed by cell lysis to produce whole cell lysates that are representative of the microdissected cell populations. RPPAs are constructed using robotic printers. Proteins of interest are detected by immunological staining methods, using validated primary antibodies and signal amplification chemistries to detect low abundance, post-translationally modified proteins. Data analysis encompasses determining spot intensity values, local area background values, normalization, and annotation of the data. Bioinformatic analyses, such as unsupervised hierarchical clustering, provide insights into similarities/dissimilarities between samples and sample groups.