Abstract
The objectives of the present study were to determine whether serum hypercholesterolemia (HC) promotes the development of spontaneous and angioplasty-induced lesions and whether amlodipine inhibits these lesions and cellular processes underlying their genesis. Rabbits were fed normal, 0.5%, or 2% cholesterol diets for 9 wk, which resulted in the development of increasing HC. After week one, balloon dilation of the abdominal aorta was performed while the thoracic aorta was not disturbed and monitored for the development of spontaneous lesions. Lesion size increased with the degree of HC and was accompanied by increased collagen synthesis and smooth muscle cell (SMC) proliferation at each site. Amlodipine (5 mg/kg po) inhibited lesion size by 50% (P < 0.01) at both sites in cholesterol-fed animals but not at angioplasty sites in animals on a normal diet. Local collagen synthesis was inhibited at both sites by amlodipine in the diet animals. The increase in HC was accompanied by a 1.7-fold increase in basal Ca2+ uptake in SMCs in the thoracic aorta, which was not altered by amlodipine, nifedipine, Ni2+, or La3+, revealing an uninhibitable calcium leak during atherogenesis. In culture, cholesterol enrichment increased SMC proliferation, collagen synthesis, and the secretion of a soluble SMC mitogen, which were inhibited by amlodipine (10−9 M). Finally, in SMC membranes, amlodipine uniquely restored the cholesterol-expanded membrane bilayer width without any effect on membrane fluidity. This study establishes a causal role between serum HC and the development of spontaneous and angioplasty-induced lesions and the ability of amlodipine to disrupt this action by a novel remodelling action on the SMC membrane.
Keywords: atherosclerosis, membranes, smooth muscle, arteries, low-density lipoproteins
Restenosis after angioplasty is a common complication of transluminal angioplasty that results from the induction of accelerated intimal hyperplasia (IH). It is generally believed that it involves the phenotypic modulation of medial smooth muscle cells (SMCs) (48), adventitial fibroblasts (34, 51), and even circulating vascular progenitor cells (18) and their subsequent migration into the intimal space, where they proliferate and deposit newly synthesized collagen. Although the primary stimulus for restenosis is the physical injury of balloon dilation to the vessel wall, the influence of various risk factors on the severity of restenosis is less clear. One of the dominant risk factors for the spontaneous development of occlusive vascular disease is hypercholesterolemia (HC), but the degree to which HC contributes to the development of IH at sites of angioplasty has not been established. We have previously shown that when blood cholesterol levels increase, SMCs of the arterial wall become enriched with unesterified (i.e., free) cholesterol (FC), which accumulates in the SMC membrane where it alters membrane function, presumably by a “swelling” effect on the structure of the membrane bilayer (4, 61). Because this effect precedes the appearance of lesions, we have suggested that enrichment of the SMC membrane with FC is a contributing factor to the cellular events underlying the genesis of atherosclerotic lesions (60, 61).
Currently, therapeutic treatment with statins has been confirmed to inhibit the progression of spontaneous lesions, but no effective treatments are available for preventing restenosis after angioplasty. In the Prospective Randomized Evaluation of Vascular Events in the Norvac Trial (PREVENT), the need for revascularization in patients with confirmed coronary artery disease decreased by 45% in patients randomized to amlodipine (Norvasc) for a 3-yr period (47), and the progression of carotid IH was abolished in these patients (3). The results of this trial suggest that, similar to statins, the antihypertensive/antianginal drug amlodipine, a third-generation dihydropyridine calcium channel blocker (CCB), also inhibits the progression of atherosclerotic lesions, independent of its blood pressure-lowering actions. Because the majority of patients undergoing transluminal angioplasty are frequently dyslipidemic, we conducted the following study to determine whether serum cholesterol influences IH and whether amlodipine has atheroprotective actions at the level of arterial SMCs. It is well established that intimal SMCs acquire a fibroproliferative (synthetic) phenotype during atherogenesis, characterized by increased cell proliferation and collagen synthesis. Accordingly, we examined the magnitude of lesion development, collagen synthesis, and SMC proliferation at sites of angioplasty-induced lesions (abdominal aorta) and spontaneous lesions (thoracic aorta) in rabbits fed a normal diet or diets containing either 0.5% or 2% cholesterol. In addition, because SMC membranes enrich with cholesterol in vivo during cholesterol feeding (4, 61), we subjected aortic SMCs in cell culture to cholesterol enrichment and measured the effects of amlodipine on collagen synthesis and SMC proliferation. We found that amlodipine inhibited IH at sites of spontaneous as well as angioplasty-induced lesions, where it inhibited collagen synthesis but not SMC proliferation. Although amlodipine failed to alter the elevated, basal calcium permeability induced in vivo by cholesterol feeding, it did reverse the structural remodeling of the SMC membrane induced by cholesterol enrichment. In cultured SMCs, amlodipine inhibited collagen synthesis, cell proliferation, and the augmented secretion of an SMC mitogen induced by cholesterol. Taken together, these observations demonstrate that cholesterol enrichment of SMCs contributes to the cellular derangements underlying the genesis of early atherosclerotic lesions and that amlodipine provides atheroprotection through a pleiotropic membrane effect unrelated to its CCB properties.
METHODS
Animals
New Zealand rabbits (male, 2.3–2.7 kg) were used in this study. The study was initiated by administering amlodipine orally (5 mg · kg−1 · day−1) to one-half the rabbits in a small quantity of peanut butter (≈50 mg) spread on lettuce. Control rabbits were fed the same preparation but without the drug (i.e., placebo). All rabbits promptly consumed all amlodipine and and/or vehicle doses. After 5–7 days, cholesterol feeding was initiated using standard rabbit chow (Purina), which consists of 30.1% protein, 5.5% fat, 21.1% fiber, and 43.3% carbohydrate or batch-matched chow supplemented with 0.5% or 2% cholesterol. One week later, all animals were anesthetized and subjected to balloon dilation of the abdominal aorta using a 4-Fr embolectomy catheter through an femoral arteriotomy. The wound was closed, and the animals were allowed to recover and were maintained on their respective drug/placebo and diet regimen for an additional 8 wk. At the end of this period, the rabbits were anesthetized with ketamine (35 mg/kg)-xylazine (5 mg/kg) intravenously, and aortic pressure was measured for 20 min through a cannula advanced down the right carotic artery to the aortic arch. After the pressure measurements, the animals were killed, and segments of the balloon-injured abdominal aorta and the noninjured thoracic aorta were isolated and harvested for study. The abdominal aorta was fixed under pressure (80 mmHg) with 2% paraformaldehyde, embedded in paraffin, sectioned, stained (hematoxylin-eosin and van Giesen stains), and analyzed morphometrically for intimal hyperplasia using Bioquant image-analysis software. IH was expressed as the intimal area-to-medial area (I/M) ratio. The thoracic aorta was opened longitudinally, stained with oil red O, and digitally imaged, and the lesion area was quantitated using NIH Image freeware. All animals were handled in accordance with Animal Lovers Against Animal Cruelty guidelines.
Tissue preparation
For calcium permeability, cell proliferation, and collagen synthesis, the vessels were dissected free of fat and connective tissue and cut into ring segments ~4 mm in width. The dissection was carried out in oxygenated physiological saline solution (PSS) of the following composition (in mmol/l): 2.5 HEPES, 2.5 Na-HEPES, 140 NaCl, 4.5 KCl, 1.0 MgCl2, 1.5 CaCl2, and 10.0 glucose, maintained at 37°C and pH 7.4, and equilibrated with 100% O2 for 60–90 min before experimentation.
Collagen synthesis was assessed in rings of the aorta labeled for 24 h with 20 µCi of [2,3-3H]proline (36 mC/mmol) (45, 46). The tissue was homogenized and dialyzed against 0.33 M calcium acetate, 25 mM Tris (pH 7.4), 0.02% NaN3, and 0.6 mM N-ethylmaleimide. Aliquots of the dialyzed samples were digested with 10 µg of bacterial collagenase III (Advance Biofactures) in albumin in a final volume of 0.5 ml at 37°C for 4 h. Proteins resistant to collagenase degradation were precipitated with the addition of an equal volume of 25% trichloracetic acid (TCA)-1.25% tannic acid. The enzyme reactions were centrifuged at 12,000 g for 5 min, and an aliquot of the supernatant was combined with scintillant and counted along with an aliquot of dialyzed sample prepared without collagenase. The collagenase-digestible protein was used to calculate the percentage of total protein synthesized as collagen by subtraction of the radioactivity in the collagenase-resistant protein from that in the total protein.
Cell proliferation was assessed using a standard [3H]thymidine uptake protocol into cellular DNA modified for freshly released SMCs. SMCs were dispersed from the medial layer of the aorta by digestion in collagenase-elastase and washed three times with minimal essential medium (MEM). After 1 h, the suspended cells (105 cells/ml) were incubated overnight at 37°C in MEM containing [3H]thymidine (0.5 µCi/ml), followed by pelleting and extraction with 10% TCA. Thymidine incorporation into DNA was expressed as counts per minute per milligram of protein in the TCA-insoluble fraction.
Calcium permeability in SMCs was assessed in ring segments using a 45Ca2+ uptake protocol as described previously (57). Briefly, because Ca2+ uptake in aortic SMCs in ring segments is linear over a 5-min period (57), segments were incubated in PSS containing 45Ca2+ (2.5 µCi/ml) at 37°C for 3 min and blotted lightly. Uptake was terminated by transferring the segments to ice-cold calcium-free PSS containing 2 mM EGTA vigorously agitated with a stream of 100% O2 for 60 min. The segments were then weighed and digested overnight in 7.5 mM EGTA, and tissue radioactivity was counted in a Beckman LS 5000TD scintillation spectrometer. Calcium uptake was determined from counts derived from the tissue 45Ca2+ fraction (in counts per minute) divided by the specific activity of the uptake media (counts · min−1 · mol−1) normalized to wet weight of the ring and expressed as micromoles per kilogram wet weight per minute.
Cell culture preparation
Early passage (passage ≤ 3) rabbit aortic SMCs were isolated as described above, grown to near confluence in MEM + 10% FBS, and growth arrested in serum-free medium. Normal and cholesterol-enriched cells were then analyzed for collagen synthesis, cell proliferation, and calcium permeability using the above methods modified for cultured cells.
Cholesterol enrichment of SMCs was performed using standard procedures (13). Briefly, the cells were arrested in serum-free medium for 3–5 days. Cholesterol-donor liposomes were applied to the cells (250 µg liposomal FC/ml), and cells were incubated for 48 h at 37°C. At the end of the incubation, the cells were washed with PBS containing 0.1% BSA to remove any adherent liposomal particles from the cell surface membranes.
Collagen protein determinations (45, 46) were performed in SMCs grown on 35-mm plates and growth arrested 3–4 days, followed by labeling with 20 µCi of [2,3-3H]proline (36 mC/mmol) per dish for an additional 24 h. The medium was collected, and proteinase inhibitors were added. The cells were trypsinized, homogenized, and dialyzed against 0.33 M calcium acetate, 25 mM Tris (pH 7.4), 0.02% NaN3, and 0.6 mM N-ethylmaleimide. Aliquots of the dialyzed samples were digested with 10 µg of bacterial collagenase III (Advance Biofactures) in albumin in a final volume of 0.5 ml at 37°C for 4 h. Proteins resistant to collagenase degradation were precipitated with the addition of an equal volume of 25% TCA-1.25% tannic acid. The enzyme reactions were centrifuged at 12,000 g for 5 min, and an aliquot of the supernatant was combined with scintillant and counted along with an aliquot of dialyzed sample prepared without collagenase. The collagenase-digestible protein was used to calculate the percentage of total protein synthesized as collagen by subtraction of the radioactivity in the collagenase-resistant protein from that in the total protein.
For membrane fluidity, steady-state anisotropy (rss; 1/membrane fluidity) was measured as previously described (2, 13). DPH was introduced into membrane vesicles by incubation of a membrane suspension (5.0 ml) with diphenylhexatriene in ethanol such that the final concentration was 0.5 mol% relative to SMC phospholipid (PL) for 15 min at 37°C. Fluorescence measurements were made with excitation at 300 nm and emission at 410 nm using a Perkin-Elmer LS50B spectrofluorimeter equipped with automatic polarizers. All fluorescence values were corrected for scattering and background fluorescence by subtraction of the values obtained from unlabeled membrane suspensions. rss was calculated using the following modified Perrin equation (63): rss = IVV − GIVH/IVV + 2GIVH, where G is calculated as G = IHV/IHH and IVH and IHH equal fluorescence intensities perpendicular and parallel, respectively, to the excitation plane. Arrhenius plots of rss as a function of temperature were constructed using rss values obtained from synthetic membranes prepared with excess membrane cholesterol. We have shown that Arrhenius plots constructed in this way demonstrate reduced membrane fluidity (increased anisotropy) with cholesterol enrichment or cooling (13).
X-ray diffraction was employed to assess membrane structure using small-angle X-ray diffraction to examine the molecular organization of isolated SMC membrane bilayers and synthetic membranes modeled after the SMC bilayer with respect of cholesterol and PL content as previously described (4, 61). Briefly, native or synthetic membranes were stacked by centrifugation on a curved substrate and elevated into the X-ray beam path so that the plane of the bilayers were parallel to the incident X-ray bream. X-ray data were collected on a one-dimensional position-sensitive electronic detector for on-line analysis. The resulting X-ray diffraction pattern was analyzed for the angle and intensity of the diffraction data. One-dimensional electron density profiles were generated from the data using Fourier analysis. X-ray diffraction was collected using a collimated, monochromatic copper Kα X-ray source, which was focused on the sample at near-grazing incidence in a temperature-controlled sample holder. The X-ray reflections were analyzed directly using a standard integration algorithm.
Synthetic membranes were prepared to examine the effects of amlodipine on the membrane bilayer structure. Lipid vesicles for these studies were prepared from dimyristoyl phosphatidylcholine (DMPC) and cholesterol reconstituted in chloroform at a cholesterol-to-PL mole ratio similar to that found in the plasma membrane of SMCs (0.8:1) isolated from atherosclerotic animals (4, 61). The lipids were dissolved in redistilled chloroform at a concentration of 10 mg/ml. Samples containing DMPC in the presence and absence of the various CCBs (≤1 µM) were dried down with a stream of nitrogen gas to a thin film in glass test tubes while being vortexed. Residual solvent was removed by vacuum. A volume of buffer (0.5 mM HEPES and 2.0 mM NaCl, pH 7.3) was added to the dried lipid preparation, yielding a final PL concentration of 5.0 mg/ml. Membrane vesicles were formed by vortexing the buffer and lipids above the thermal phase transition temperature for 3 min. Oriented membrane samples were prepared by centrifugation, as described previously by us for native SMC membrane samples (4, 61).
Lipid and protein determinations were made in tissue and membranes after their extraction in chloroform-methanol-water following the method of Bligh and Dyer (1). The chloroform phase was obtained and dried under nitrogen. Total and (unesterified) FC mass was determined by gas-liquid chromatography (19, 28) using cholesterol methyl ether as an internal standard. Cholesteryl ester is obtained by subtraction of FC from total cholesterol. PL mass was assessed using a standard PL phosphorus assay as previously described (4) using inorganic phosphate as a standard. Protein determinations were performed using the method of Lowry (35).
Statistics
Values represent means ± SE and were compared by unpaired t-test or ANOVA for repeated measures followed by the Newman-Keuls post hoc analysis, as indicated. P values <0.05 were considered statistically significant. In studies performed in vivo, n refers to the number of animals studied. In studies performed in cell culture, n refers to the number of experiments performed where each experiment was performed in triplicate and the resulting average of the triplicates was used for n = 1.
RESULTS
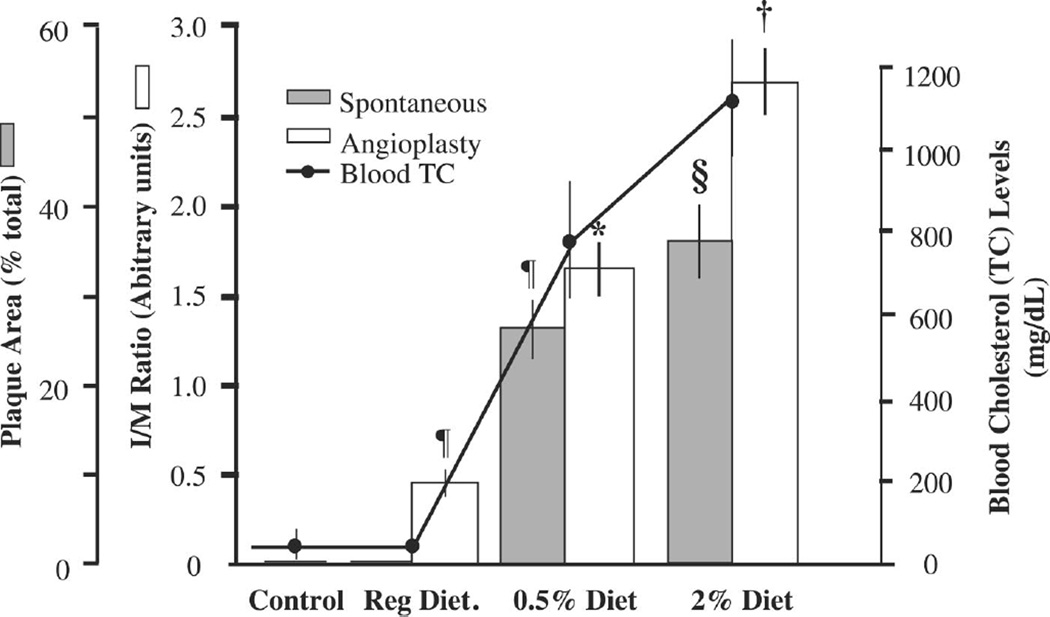
In the thoracic aorta, spontaneous atherosclerotic lesions were not present in animals maintained on the control diet, whereas the 0.5% cholesterol diet induced lesions over 27% (26.69 ± 3.23) of the intimal surface, and the 2% diet induced lesions over 37% (36.53 ± 4.46) of the intimal surface (Fig. 1). In the abdominal aorta, balloon angioplasty induced significant IH in animals fed the control diet (0.46 ± 0.04 vs. 0.00 ± 0.00 units, P < 0.001), and this increased 3.7-fold (0.46 ± 0.05 vs. 1.68 ± 0.15 units, P < 0.01) in animals fed the 0.5% diet and 5.9-fold (0.46 ± 0.05 vs. 2.70 ± 0.18 units, P < 0.01) in animals fed the 2% cholesterol diet. The magnitude of lesion development increased with increasing cholesterol content in the diet (control: 44 ± 6 mg/dl; 0.5%: 833 ± 159 mg/dl; and 2%: 1,109 ± 124 mg/dl).
Fig. 1.
Size of spontaneous lesions (shaded bars) as well as angioplasty-induced intimal hyperplasia (open bars) increases with increasing blood cholesterol achieved by feeding animals a cholesterol diet supplemented with either 0.5% or 2% cholesterol. TC, total cholesterol; I/M ratio, intimal area-to-medial area ratio. ¶P < 0.001, 0.5% diet vs. control diet; §P < 0.05, 2% diet vs. 0.5% diet; *P < 0.01, 0.5% diet vs control diet; †P < 0.01, 2% diet vs. 0.5% diet. n ≥ 6 animals. Statistical analysis was by ANOVA.
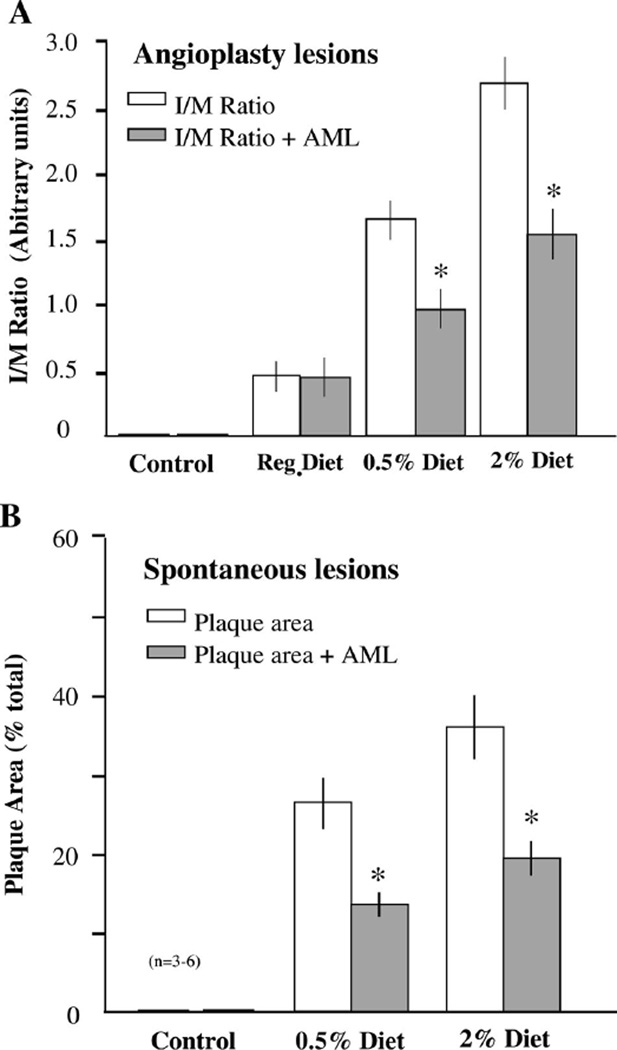
Animals pretreated with amlodipine demonstrated an ~41% inhibition in IH at the sites of angioplasty in the abdominal aorta (1.64 ± 0.14 vs. 0.96 ± 0.16 IM ratio, P < 0.05) and 42% (2.67 ± 0.19 vs. 1.53 ± 0.21 IM ratio, P < 0.05) in the (Fig. 2A). Similarly, amlodipine treatment inhibited the development of spontaneous lesions in the thoracic aorta by 49% (26.8 ± 3.2 vs. 13.8 ± 1.69, P < 0.05) and 46% (36.4 ± 4.4 vs. 19.7 ± 2.3, P < 0.05) in the animals fed the 0.5% and 2% cholesterol diet, respectively (Fig. 2B). Interestingly, amlodipine had no effect on IH in the animals with low blood cholesterol levels that were maintained on the control diet. This inhibition of lesions was not associated with any change in mean blood pressure (control: 71.0 ± 2.9 vs. 67.8 ± 3.2 mmHg without vs. with AML; cholesterol fed: 67.2 ± 2.1 vs. 65.9 ± 3.8 mmHg without vs. with AML). A similar lack of blood pressure lowering by amlodipine in normotensive rats (41), rabbits (17), monkeys (32), and humans (47) has been reported previously.
Fig. 2.
A: oral amlodipine (AML; shaded bars) suppresses the development of angioplasty-induced intimal hyperplasia in the abdominal aorta by ~40% compared with placebo (open bars) in hypercholesterolemic animals regardless of the magnitude of increase in serum cholesterol levels. Interestingly, AML had no effect on intimal hyperplasia in animals fed the normal (cholesterol free) diet. This finding suggests a dependence of the AML effect on enrichment of smooth muscle cells (SMCs) with cholesterol. B: oral AML inhibited the development of spontaneous lesions in the thoracic aorta by ~45% in hypercholesterolemic animals regardless of the magnitude of increase in serum cholesterol levels. *P < 0.05, AML treated vs. placebo. n = 3–6 animals. Statistical analysis was by ANOVA.
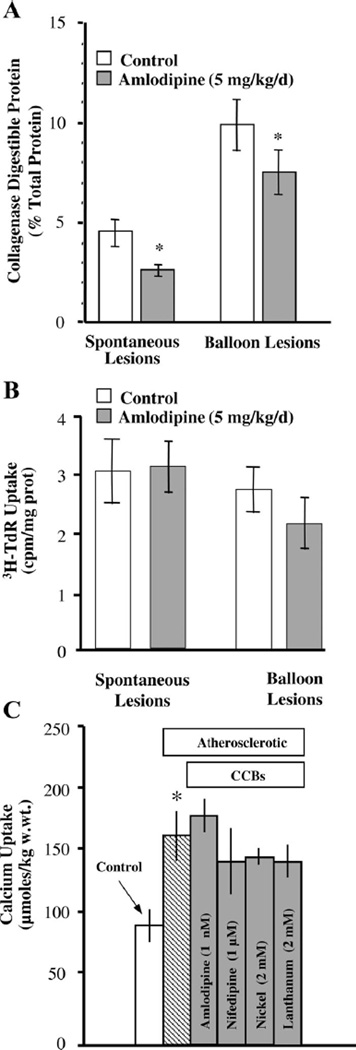
The cellular processes that amlodipine disrupts were assessed in aortic SMCs in animals fed cholesterol and treated with or without amlodipine. Collagen synthesis in vivo was inhibited by 41% by amlodipine therapy (4.6 ± 0.8 vs. 2.6 ± 0.3% total protein, P < 0.05) at sites of spontaneous lesions in the thoracic aorta, whereas it was reduced by 23% (9.8 ± 1.3 vs. 7.5 ± 1.1% total protein, P < 0.05) at sites of angioplasty in the abdominal aorta (Fig. 3A). SMC proliferation was not altered by amlodipine at sites of spontaneous lesions in the thoracic aorta, whereas there was an average reduction of 18% at the sites of angioplasty in the abdominal aorta, but this reduction did not reach statistical significance (Fig. 3B). Calcium uptake in medial SMCs of the thoracic aorta was elevated in the cholesterol-fed (0.5%) animals by 82% (88 ± 13 vs. 160 ± 20 µmol/kg wet wt). Interestingly, neither amlodipine, nifedipine (1 µM), nor the inorganic CCBs Ni2+ (2 mM) or La3+ (2 mM) were able to alter this augmented basal calcium permeability (Fig. 3C). This observation is consistent with the suggestion that an uninhibitable calcium “leak” pathway is expressed in the SMC membrane during the early developmental period of atherosclerotic lesions.
Fig. 3.
A: collagen synthesis is increased at sites of spontaneous and angioplasty-induced lesions in cholesterol-fed (0.5%) rabbits, and oral AML (shaded bars) inhibited collagen synthesis at both sites. B: however, AML failed to significantly inhibit SMC proliferation at either site. C: hypercholesterolemia in rabbits on the 0.5% diet increased calcium uptake by 82% in the thoracic aorta (hatched bar), but neither the organic (amlodipine, nifedipine) nor inorganic (Ni2+, La3+) calcium channel blockers (CCBs; shaded bars) inhibited this increase, demonstrating the development of an uninhibitable calcium “leak” in the early atherogenic period. *P < 0.05, atherosclerosis vs. control. n = 7 animals. Statistical analysis was by unpaired Student’s t-test (A and B) and by ANOVA (C).
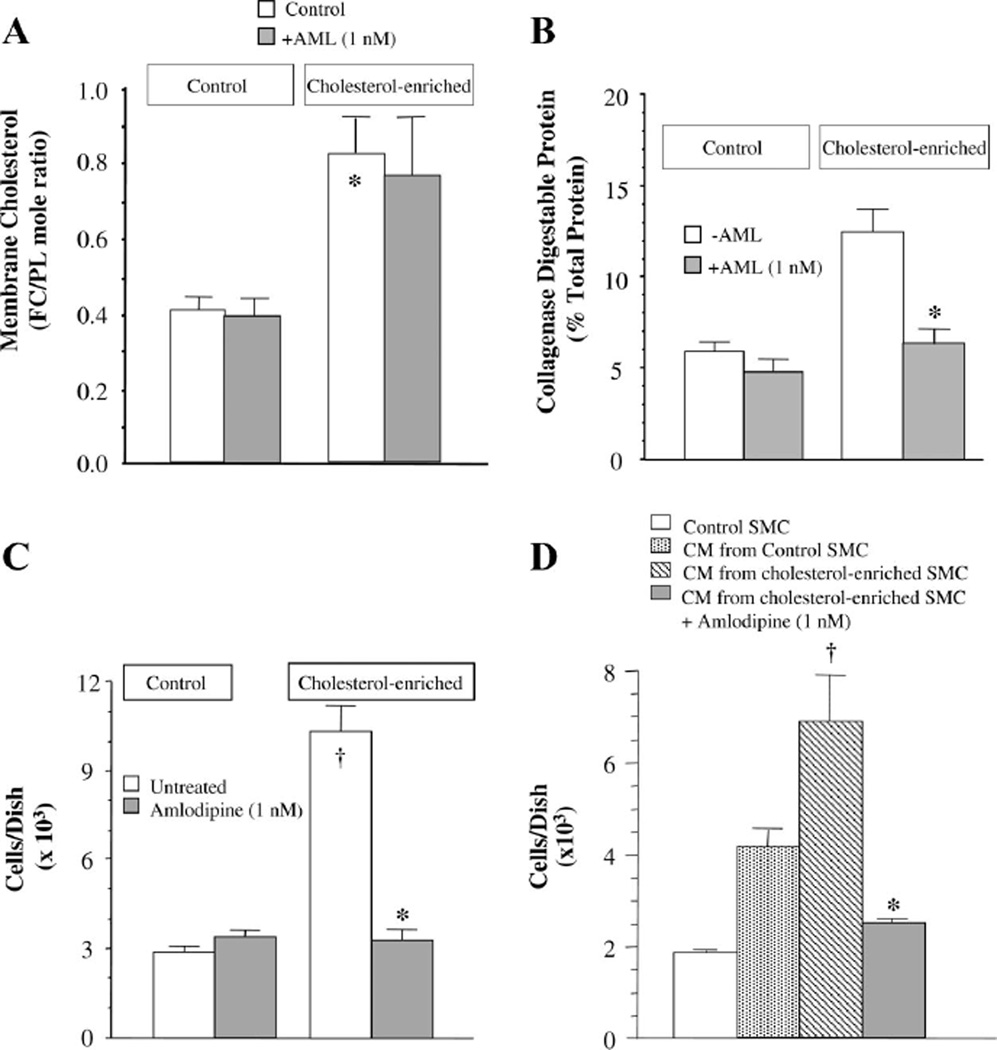
Because aortic SMCs enrich with cholesterol during the early period of atherogenesis in cholesterol-fed rabbits (4), we examined the effects of enrichment of SMCs in culture with exogenous cholesterol on collagen synthesis, cell proliferation, and calcium uptake and the degree to which amlodipine inhibits these processes. Cholesterol enrichment increased the SMC membrane FC content (0.41 ± 0.05 vs. 0.82 ± 0.10 FC-to-PL mole ratio, P < 0.01), and amlodipine (1 nM) had no effect on this (Fig. 4A). Cholesterol enrichment also increased collagen synthesis 2.1-fold (5.95 ± 0.6 vs. 12.4 ± 1.3% collagenase digestible protein, P < 0.01), and amlodipine (1 nM) abolished this increase but was without effect in control SMCs (Fig. 4B). Cholesterol enrichment increased SMC proliferation by 3.6-fold (2.86 ± 0.22 vs. 10.37 ± 0.88 × 103 cells/cm2), and amlodipine (1 nM) abolished this increase as well but was without effect in control SMCs (Fig. 4C).
Fig. 4.
A: in cultured SMCs, enrichment with exogenous cholesterol increased the free cholesterol (FC) content in the cell membrane by 2-fold. Incubation of the cells with AML (1 nM) had no effect on membrane cholesterol content in control cells or cells subjected to cholesterol enrichment. PL, phospholipid. *P < 0.01, cholesterol enriched vs. control. n = 5–7. B: cholesterol enrichment increased SMC collagen synthesis. Whereas AML had no effect on control SMCs, it abolished the cholesterol-induced increase in synthesis. *P < 0.01, cholesterol-enriched SMCs treated with AML vs. not treated. n = 4. C: cholesterol enrichment increased SMC proliferation by 3.6-fold. Whereas AML had no effect on control SMCs, it abolished the cholesterol-induced increase in cell proliferation. †P < 0.01, cholesterol enriched vs. control; *P < 0.05, cholesterol-enriched SMCs treated with AML vs. not treated. n = 6. D: culture medium (CM) conditioned with cholesterolenriched SMCs had twice the mitogenic activity on SMC proliferation as medium conditioned with growth-arrested control SMCs. AML treatment of cholesterol-enriched SMCs abolished the mitogenic activity in the conditioned medium. *P < 0.01, medium from cholesterol-enriched SMCs treated with AML vs. medium from cholesterol-enriched SMCs not treated with AML. n = 3. In C and D, cells were seeded at a density of 3.5 × 103 cells/cm2. Statistical analysis was by unpaired Student’s t-test (A–C) and by ANOVA (D).
Recent studies have revealed that amlodipine inhibits SMC proliferation by inhibiting the activation of c-Fos (62). To evaluate other potential pathways for this antiproliferative effect, we examined the ability of cholesterol enrichment of SMC to induce the secretion of a soluble SMC mitogen and the degree to which amlodipine might be able to inhibit this activity. Confluent SMCs in T-25 culture flasks were subjected to cholesterol enrichment ± amlodipine (1 nM) for 6 h, washed three times with 0.1% BSA, and incubated overnight in serum-free MEM to generate conditioned medium. Separate SMCs seeded at subconfluence and growth arrested in 12-well plates were rinsed free of medium and incubated with the conditioned medium for 48 h, followed by cell counting using a hemocytometer. Conditioned medium from cholesterol-enriched SMC induced marked proliferation (>3.6-fold) of SMCs (1.9 ± 0.1 vs. 6.9 ± 1.0 × 103 cells/cm2, P < 0.01; Fig. 4D). In addition, SMCs incubated with medium conditioned by normal cells also induced SMC proliferation, but this effect was about half that seen when SMCs were incubated with medium conditioned with cholesterol-enriched cells. Interestingly, the proliferative action of medium conditioned by the cholesterol-enriched cells was inhibited by 88% (6.9 ± 1.0 vs. 2.5 ± 0.1 × 103 cells/cm2, P < 0.01) when the cells were treated with amlodipine during the cholesterol enrichment procedure. This observation suggests that cholesterol enrichment of SMCs induces the secretion of an SMC mitogen and that amlodipine inhibits the synthesis and/or secretion of this mitogen.
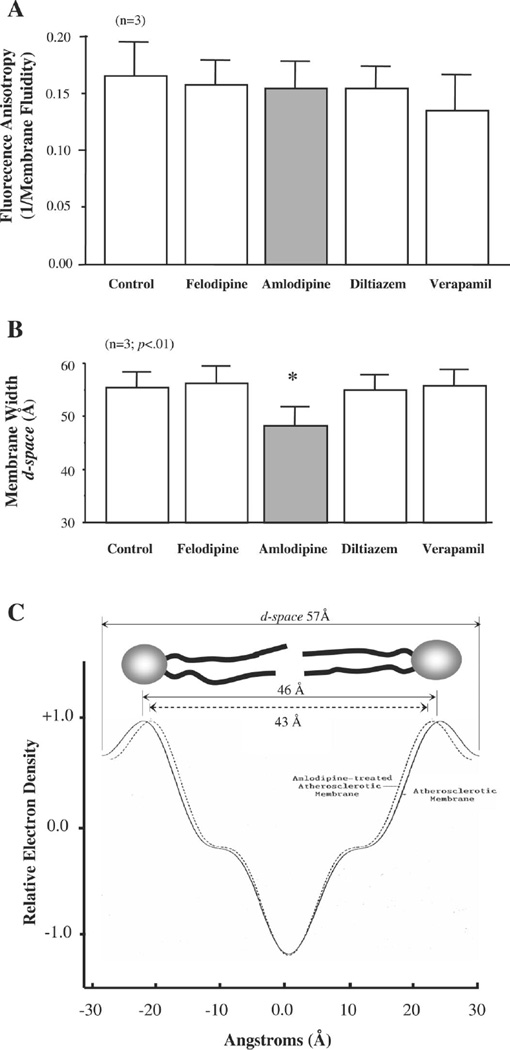
Because amlodipine has been shown to avidly partition into lipid bilayers (37), we examined its effects on membrane lipid dynamics and structure using a combination of fluorescence anisotropy (1/membrane fluidity) and small-angle X-ray diffraction. In synthetic lipid bilayers formed from cholesterol and DMPC (0.8:1 mole ratio), membrane fluidity was unaffected by the addition of any of the organic CCBs to the bilayers (Fig. 5A). However, amlodipine, but not felodipine, diltiazem, or verapamil, reduced membrane width as reflected by the 13% decrease in d-space (lipid-water interface on one side of the membrane leaflet to the lipid-water interface on the opposing leaflet; 55.2 ± 2.9 vs. 48.0 ± 3.6 Å, P < 0.05) in these model membranes (Fig. 5B). In native membranes isolated from aortic SMCs of cholesterol-fed rabbits, the addition of amlodipine to the membrane pellet also decreased membrane width by 5.6%, as assessed from the d-space measurements (57.6 vs. 54.4 Å) as well as the PL head group separation by 4.9% (46.4 vs. 44.2 Å; Fig. 5C). These data clearly demonstrate that amlodipine has the ability to remodel the “swollen” membrane of atherosclerotic SMCs that we have previously described in Refs. 4 and 61 and restore it to normal values.
Fig. 5.
A: CCBs fail to alter membrane fluidity in FC/DMPC membrane vesicles. B: AML, but not felodipine, diltiazem, or verapamil, reduced membrane width. *P < 0.05, AML treated vs. control. n = 3. C: AML treatment reduced membrane width by 5.6% and phosphorus head group separation by 4.9% in native membranes isolated from SMCs freshly harvested from an atherosclerotic aorta. All CCBs were used at a concentration of 0.1 µM. Statistical analysis was by ANOVA (A and B).
DISCUSSION
The severity of atherosclerotic vascular disease is well known to correlate with the number and severity of risk factors. The tissue basis for the expansion of lesions involves increased matrix synthesis by SMCs, increased lipid retention to this matrix, and increased cellularity due, in large part, to increased SMC proliferation (65). Whereas all of these are accelerated in angioplasty-induced lesions, the degree to which HC, in general, and cholesterol, in particular, contributes to lesion formation at sites of spontaneous or angioplasty territories has not been well studied. At sites of spontaneous lesions, the primary insult to the vessel wall is increased exposure to circulating cholesterol-rich lipoproteins, whereas in angioplasty-induced lesions, the physical injury caused by balloon dilation induces IH independent of blood cholesterol levels. In the absence of HC, these lesions regress and resolve spontaneously (8), but in the presence of HC they not only sustain but progress to larger lesions. In clinical settings, typically the two separate insults simultaneously combine to drive the progression of these lesions. In the present study, we confirm that plaque size at sites of spontaneous lesions increases with increasing blood cholesterol levels. These findings are consistent with the concept that the severity of atherosclerosis is determined, in part, by the level of HC, as has been inferred from epidemiological studies for spontaneous lesions (25, 26). However, the marked augmentation of angioplasty-induced lesions with increasing blood cholesterol concentrations is a new finding. Clinical studies addressing the relationship between circulating lipoprotein levels and restenosis are not clear. For example, elevated apolipoprotein B correlated positively with restenosis (9), as did high preprocedural non-HDL cholesterol levels (6). In addition, reduced HDL levels also correlated positively with restenosis (9, 23). Low HDL levels would be expected to result in reduced reverse cholesterol transport and increase tissue (vessel wall) cholesterol levels. Unfortunately, reducing LDL levels with statins failed to alter restenosis rates (16). However, dramatic reduction in LDL levels with plasma apheresis has been shown to decrease restenosis rates (29%) compared with controls (42%), but these differences failed to achieve statistical significance, perhaps due to the small sample size (n ≤ 17) and relatively few patients with HC (<27%) enrolled in this study (66). To examine the potential for elevated blood cholesterol to augment the development of lesions at the cellular level, we show that cholesterol enrichment of growth-arrested SMCs in culture is sufficient by itself to stimulate SMC proliferation and collagen synthesis, suggesting the novel concept that cholesterol accumulation in SMC contributes to the cellular alterations underlying the early pathogenic events leading to the development of atherosclerotic lesions and may contribute importantly to the expansion of angioplasty-induced lesions if not also spontaneous lesions.
In the present study, oral therapy with amlodipine had no effect on IH when blood cholesterol levels were low, whereas it has considerable inhibitory actions (≈50%) when blood cholesterol levels were elevated. This suggests that the cellular events underlying the physical injury of angioplasty in this model are fundamentally different than those that occur in the setting of HC and that amlodipine has little effect on these processes. Moreover, it also shows an effect of amlodipine that is selective for a “lipid injury” in HC. Amlodipine has been shown to inhibit the genesis of spontaneous lesions in animal models of atherosclerosis (31, 42, 52, 62) as well as in humans (3, 30, 47). Although inhibition by amlodipine of angiographic restenoisis after angioplasty in the Coronary Angioplasty Amlodipine Restenosis Study trial did not reach statistical significance, the need for revascularization and cardiovascular mortality was significantly decreased (24). Notwithstanding, a meta-analysis of this trial combined with the Nisoldipine in Coronary Artery Disease in Leuven (NICOLE) trial did reveal a significant decrease in angiographic restenosis (12). Our studies confirm these observations as well as identify new and novel cellular pathways that amlodipine may be disrupting.
In vivo, oral amlodipine administration inhibited collagen synthesis at sites of both spontaneous and angioplasty-induced lesions. This observation supports the findings of Roth et al. (49), who demonstrated that amlodipine inhibits the expression of collagen mRNA in human vessels. Amlodipine had no effect on SMC proliferation at sites of spontaneous lesions, whereas the reduction in proliferation observed at the angioplasty sites (≈20%) failed to achieve statistical significance. Difficulty observing an effect of amlodipine on SMC proliferation in vivo is not surprising because it has been demonstrated that SMC proliferation occurs early (<5 wk) in the development of stretch-induced lesions (7, 54), whereas our study examined proliferation 10 wk postdiet/injury. It is particularly interesting, however, that neither the organic CCBs (amlodipine or nifedipine), or inorganic CCBs (Ni2+, La3+) were able to block the increase in basal calcium permeability seen in SMCs in the atherosclerotic aorta. This observation demonstrates that the early atherogenic period in arterial SMCs is associated with the expression of an uninhibitable Ca2+ leak pathway in the SMC membrane. Importantly, we have previously shown that amlodipine indeed has dose-dependent inhibitory activity on Ca2+ uptake in normal aortic SMCs, an action that is associated with an IC50 of ~3 nM (60), similar to that seen with other CCBs. Despite the development of an uninhibitable Ca2+ leak, amlodipine has clear atheroprotective actions, supporting the suggestion that this effect is mediated by properties independent of its ability to block calcium uptake.
In SMCs growth arrested in culture, cholesterol delivery to the cells resulted in the enrichment of the SMC membrane with cholesterol as we have previously shown (13, 61). This resulted in a marked increase in collagen synthesis, cell proliferation, and the secretion of an SMC mitogen. These findings are consistent with the concept that cholesterol activates cellular inflammatory pathways that induce the modulation of growth-arrested SMCs to a fibroproliferative phenotype. They further suggest that the proliferative effect is mediated by activation of an autocrinic loop through the secretion of a soluble growth factor. Although we were unable to identity the growth factor released, it is clear that cholesterol enrichment either increased the secretion of the same growth factor that we (Fig. 4D) and others have seen in cultured SMCs (29, 50, 58) or it releases a different growth factor. In either case, preincubation of SMCs with amlodipine prevented its release. Amlodipine has been shown to inhibit SMC proliferation induced by serum, thrombin, and FGF by a pathway that is independent of its CCB properties (56). Antiproliferative actions of a variety of CCBs have been described previously (15, 20), and a CCB-independent effect has been inferred from antiproliferative actions of lercanidipine and verapamil using their inactive enantiomeres (10, 44). In our study, we observed an uninhibitable calcium leak in medial SMCs of the atherosclerotic aorta. Augmented calcium influx and activation of p42/p44 MAPK (MAPK/ERK1/2) appear to be involved in the regulation of cell cycle progression from G1 to S and thus cell proliferation (36, 53, 56, 64). However, the ability of amlodipine to inhibit the modulation of SMCs to the fibroproliferative phenotype in our study, despite its lack of effect on the calcium leak, suggests an upstream effect, perhaps at the cell membrane.
Amlodipine is strongly lipophilic and avidly partitions into cell membranes, where it has a long half-life (37). Taking advantage of this property, we examined its ability to alter membrane lipid dynamics (fluidity) and organization in PL bilayers modeled after SMC membranes isolated from the atherosclerotic aorta (4, 61). We found that amlodipine restored membrane bilayer width back toward normal on its addition to cholesterol-rich membrane bilayers. A similar result was obtained when amlodipine was added to native SMC membranes isolated from HC/atherosclerotic rabbits. Surprisingly, amlodipine had no effect on membrane fluidity or FC content; instead, it restored membrane structure independent of any effect on cholesterol content or lipid dynamics. This finding was unexpected because cholesterol accumulation in membranes increases intermolecular packing in the fatty acyl chain region of the bilayer, and thus membrane viscosity, as shown previously by us (13). We have argued that because cholesterol packing into this region of the bilayer restricts angular motion of the PL fatty acyl chains, which results in an obligate increase in the time-averaged long-axis length of the PL molecule, expansion (“swelling”) of the lipid bilayer occurs (59, 61). However, the present data clearly show that amlodipine uncouples the condensing effect of cholesterol from its ability to increase membrane bilayer width, thereby raising theoretical questions regarding the interplay between lipid dynamics and lipid structure. This apparent discrepant observation notwithstanding, we report here a drug effect that may be coupled to therapeutic atheroprotection at the level of reordering the organization of the membrane lipid bilayer.
Although we demonstrate an effect of amlodipine potentially mediated at the level of the cell membrane, it is important to note that other investigators have found several alternative CCB-independent effects of amlodipine that may also explain its atheroprotective actions. For example, amlodipine increases nitric oxide release from endothelial cells through a pathway that appears to be mediated by bradykinin (69) and/or Akt activation (33). In addition, amlodipine has antioxidant actions in rat (27), nonhuman primates (32), and rabbit (62) models of atherosclerosis as well as in model membranes (40). Other studies have shown that it decreases smooth muscle collagen mRNA levels (49) and c-Fos expression in rats (56). It also has effects on circulating elements including reducing levels of oxidized LDL (oxLDL) in humans (38) and decreasing activation of circulating monocytes in mice (27). Taken together, amlodipine has several pleiotropic actions that may work alone or, more likely, in combination to inhibit the activation of inflammatory processes, as shown by others (5, 27). Indeed, amlodipine markedly inhibits the induction of proinflammatory cytokines in SMCs in vivo and in cell culture, including IL-1, IL-6, TNF-α, and superoxide anion (5) as well as macrophage chemoattractant protein 1, local angiotensin-converting enzyme, and Rho (27). Taken together with the results of the present study, these data strongly support an anti-inflammatory mechanism of action for atheroprotection with amlodipine. Considering its inability to alter the elevated calcium uptake in SMCs of the atherosclerotic aorta, it appears unlikely that its CCB effect contributes significantly to its atheroprotective actions. Interestingly, this may explain why CCBs have failed to convincingly demonstrate atheroprotection (21) as a class action, whereas amlodipine and lacidipine (11, 68), a new CCB closely related to amlodipine, do show this therapeutic property.
Although HC has been long known to induce the development of atherosclerotic lesions in humans and a variety of animal models, the cellular basis for this action has been largely attributed to the formation of oxLDL and oxidative injury to the endothelium (55). In this context, blood cholesterol therefore is generally viewed merely as a “marker” for LDL levels and thus oxLDL-mediated oxidative stress. Although there appears to be little doubt that oxidative stress plays an important role in atherogenesis, we provide data in this study consistent with the suggestion that cholesterol enrichment of SMCs may contribute importantly to the cellular events that initiate early cellular derangements leading to the development of atherosclerotic lesions. The concept of an atherogenic stimulus secondary to excess membrane cholesterol in vascular cells identifies a potentially redundant pathway to atherogenesis independent of oxidative stress and may explain the relative ineffectiveness of supplemental dietary antioxidants to provide atheroprotection in human studies (14, 43, 64a, 67).
In summary, we show that amlodipine appears to stabilize SMCs in the quiescent (“contractile”) phenotype, thus inhibiting their modulation to the atherosclerotic (“synthetic”) phenotype. We suggest that its novel ability to restore the altered cell membrane structure that occurs early in the atherogenic period underlies this effect. It is widely assumed that atheroprotective pharmacotherapy is only available through statins or agents that improve dyslipidemias. As shown in this study, and reviewed recently (39), amlodipine inhibits the development of atherosclerotic lesions in humans and a variety of animal models, without effects on blood pressure or blood lipids. We suggest that amlodipine has the potential for atheroprotective pharmacotherapy independent of the presence of hypertension or dyslipidemia.
Acknowledgments
GRANTS
Support for this project was provided in part by National Heart, Lung, and Blood Institute Grants R01-HL-66273 and P01-HL-07443, a grant from Pfizer, the American Heart Association, and the Churchman Postdoctoral Fellowship Fund.
REFERENCES
- 1.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 2.Boesze-Battaglia K, Schimmel R. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. J Exp Biol. 1997;200:2927–2936. doi: 10.1242/jeb.200.23.2927. [DOI] [PubMed] [Google Scholar]
- 3.Byington R, Riley W, Booth D, Herrington D, Hunninghake D, Mallon S, Miller M, Ramanathan K, Werns S, Furberg C, Pitt B. Effect of amlodipine on progression of carotid atherosclerosis in patients with documented heart disease. Am J Hypertens. 1999;12:42A–43A. [Google Scholar]
- 4.Chen M, Mason RP, Tulenko TN. Atherosclerosis alters composition, structure and function of arterial smooth muscle plasma membranes. Biochim Biophys Acta. 1995;1272:101–112. doi: 10.1016/0925-4439(95)00073-d. [DOI] [PubMed] [Google Scholar]
- 5.Chou T, Yang S, Pei D. Amlodipine inhibits pro-inflammatory cytokines and free radical production and inducible nitric oxide synthase expression in lipopolysaccharide/interferon-gamma-stimulated cultured vascular smooth muscle cells. Jpn J Pharmacol. 2002;89:157–163. doi: 10.1254/jjp.89.157. [DOI] [PubMed] [Google Scholar]
- 6.Cipollone F, Fazia M, Iezzi A, Pini B, Costantini F, DeCesare D, Paloscia L, Materazzo G, D’Annunzio E, Bucciarelli T, Vecchiet J, Chiarelli F, Cuccurullo F, Mezzetti A. High preprocedural non-HDL cholesterol is associated with enhanced oxidative stress and monocyte activation after coronary angioplasty: possible implications in restenosis. Heart. 2003;89:773–779. doi: 10.1136/heart.89.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clowes A, Schwartz S. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985;56:139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- 8.Consigny P, Tulenko T, Nicosia R. Acute effects of angioplasty on vascular smooth muscle. Arteriosclerosis. 1986;6:265–276. doi: 10.1161/01.atv.6.3.265. [DOI] [PubMed] [Google Scholar]
- 9.Cooke T, Sheahan R, Foley D, Reilly M, D’Arcy G, Jauch W, Gibney M, Gearty G, Crean P, Walsh M. Lipoprotein(a) in restenosis after percutaneous transluminal coronary angioplasty and coronary artery disease. Circulation. 1994;89:1593–1598. doi: 10.1161/01.cir.89.4.1593. [DOI] [PubMed] [Google Scholar]
- 10.Corsini A, Bonfatti M, Quarato P, Accomazzo M, Raiteri M, Sartani A, Testa R, Nicosia S, Paoletti R, Fumagalli R. Effect of the new calcium antagonist lercanidipine and its enantiomers on the migration and proliferation of arterial myocytes. J Cardiovasc Pharmacol. 1996;28:687–694. doi: 10.1097/00005344-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Cristofori P, Lanzoni A, Quartaroli M, Pastorino A, Zancanaro C, Cominacini L, Gaviraghi G, Turton J. The calcium-channel blocker lacidipine reduces the development of atherosclerotic lesions in the apoE-deficient mouse. J Hypertens. 2000;18:1429–1436. doi: 10.1097/00004872-200018100-00010. [DOI] [PubMed] [Google Scholar]
- 12.Dens J, Desmet W, Piessens J. An updated meta-analysis of calcium-channel blockers in the prevention of restenosis after coronary angioplasty. Am Heart J. 2003;145:404–408. doi: 10.1067/mhj.2003.171. [DOI] [PubMed] [Google Scholar]
- 13.Gleason MM, Medow MS, Tulenko TN. Excess membrane cholesterol alters calcium movements, cytosolic calcium levels, and membrane fluidity in arterial smooth muscle cells. Circ Res. 1991;69:216–227. doi: 10.1161/01.res.69.1.216. [DOI] [PubMed] [Google Scholar]
- 14.Henneckens C, Buring J, Manson J, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 15.Hirata A, Igarashi M, Yamaguchi H, Suwabe A, Daimon M, Kato T, Tominaga M. Nifedipine suppresses neointimal thickening by its inhibitory effect on vascular smooth muscle cell growth via a MEK-ERK pathway coupling with Pyk2. Br J Pharmacol. 2000;131:1521–1530. doi: 10.1038/sj.bjp.0703730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horlitz M, Sigwart U, Niebauer J. Fighting restenosis after coronary angioplasty: contemporary and future treatment options. Int J Cardiol. 2002;83:199–205. doi: 10.1016/s0167-5273(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 17.Hoshida S, Yamashita N, Kuzuya T, Hori M. Reduction in infarct size by chronic amlodipine treatment in cholesterol-fed rabbits. Atherosclerosis. 1998;138:163–170. doi: 10.1016/s0021-9150(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Davison F, Zhang Z, Xu Q. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation. 2003;108:3122–3127. doi: 10.1161/01.CIR.0000105722.96112.67. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa TT, MacGee J, Morrison JA, Glueck CJ. Quantitative analysis of cholesterol in 5 to 20 microliters of plasma. J Lipid Res. 1975;15:286–291. [PubMed] [Google Scholar]
- 20.Jackson C, Schwartz S. Pharmacology of smooth muscle cell replication. Hypertension. 1992;20:713–736. doi: 10.1161/01.hyp.20.6.713. [DOI] [PubMed] [Google Scholar]
- 21.Jackson CL, Bush RC, Bowyer DE. Mechanism of antiatherogenic action of calcium antagonists. Atherosclerosis. 1989;80:17–26. doi: 10.1016/0021-9150(89)90063-4. [DOI] [PubMed] [Google Scholar]
- 23.Johansson S, Wiklund O, Karlsson T, Hjalmarson A, Emanuelsson H. Serum lipids and lipoproteins in relation to restenosis after coronary angioplasty. Eur Heart J. 1991;12:1020–1028. doi: 10.1093/eurheartj/12.9.1020. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen B, Simonsen S, Endresen K, Forfang K, Vatne K, Hansen J, Webb J, Buller C, Goulet G, Erikssen J, Thaulow E. Restenosis and clinical outcomes in patients treated with amlodipine after angioplasty: results from the Coronary Angioplasty Amlodipine REStenosis Study (CAPARES) J Am Coll Cardiol. 2000;35:592–599. doi: 10.1016/s0735-1097(99)00599-9. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB. High density lipoproteins: epidemiologic profile and risks of coronary artery disease. Am J Cardiol. 1983;52:9B–13B. doi: 10.1016/0002-9149(83)90649-5. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Neaton JD, Wentworth D. Overall coronary heart disease mortality rate in relation to major risk factors in 325,348 men screened for the MRFIT. Am Heart J. 1986;112:825–836. doi: 10.1016/0002-8703(86)90481-3. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka C, Egashira K, Ishibashi M, Inoue S, Ni W, Hiasa K, Kitamoto S, Usui M, Takeshita A. Novel anti-inflammatory actions of amlodipine in a rat model of arteriosclerosis induced by long-term inhibition of nitric oxide synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H768–H774. doi: 10.1152/ajpheart.00937.2002. [DOI] [PubMed] [Google Scholar]
- 28.Klansek J, Yancey PG, St Clair RW, Fisher RT, Johnson WJ, Glick JM. Cholesterol quantitation by GLC: artifactual formation of short chain steryl esters. J Lipid Res. 1995;36:2261–2266. [PubMed] [Google Scholar]
- 29.Koo B, Kim D. Factor Xa induces mitogenesis of vascular smooth muscle cells via autocrine production of epiregulin. J Biol Chem. 2003;278:52578–53586. doi: 10.1074/jbc.M310007200. [DOI] [PubMed] [Google Scholar]
- 30.Koshiyama H, Tanaka S, Minamikawa J. Effect of calcium channel blocker amlodipine on the intimal-medial thickness of carotid arterial wall in type 2 diabetes. J Cardiovasc Pharmacol. 1999;33:894–896. doi: 10.1097/00005344-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Kramsch DM. Limits of lipid-lowering therapy: the benefits of amlodipine as an anti-atherogenic agent. J Hum Hypertens. 1995;9:S3–S9. [PubMed] [Google Scholar]
- 32.Kramsch DM. Limits of lipid-lowering therapy: the potential benefits of amlodipine as an anti atherosclerotic agent. Int J Cardiol. 1997;62:5119–5124. doi: 10.1016/s0167-5273(97)00249-0. [DOI] [PubMed] [Google Scholar]
- 33.Lenasi H, Kohlstedt K, Fichtlscherer B, Mulsch A, Busse R, Fleming I. Amlodipine activates the endothelial nitric oxide synthase by altering phosphorylation on Ser1177 and Thr495. Cardiovasc Res. 2003;59:844–853. doi: 10.1016/s0008-6363(03)00505-4. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Chen S, Oparil S, Chen Y, Thompson J. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 35.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Magnier-Gaubil C, Herbert JM, Quarck R, Papp B, Corvazier E, Wuytack F, Lévy-Tolédano S, Enouf J. Smooth muscle cell cycle and proliferation: relationship between calcium influx and sarcoendoplasmic reticulum Ca2+-ATPase regulation. J Biol Chem. 1996;271:27788–27794. doi: 10.1074/jbc.271.44.27788. [DOI] [PubMed] [Google Scholar]
- 37.Mason R, Campbell S, Wang S, Herbette L. Comparison of location and binding for the positively charged 1,4-dihydropyridine calcium channel antagonist amlodipine with uncharged drugs of this class in cardiac membranes. Mol Pharmacol. 1989;36:634–640. [PubMed] [Google Scholar]
- 38.Mason R, Jeffers B, Preston G, Jacob R, Buch J, Walter M. Low-density lipoprotein oxidation biomarkers predict cardiovascular events in the PREVENT study (Abstract) Circulation. 2003;108:1460A. [Google Scholar]
- 39.Mason R, Marche P, Hintze T. Novel vascular biology of thirdgeneration L-type calcium channel antagonists: ancillary actions of amlodipine. Arterioscler Thromb Vasc Biol. 2003;23:2155–2163. doi: 10.1161/01.ATV.0000097770.66965.2A. [DOI] [PubMed] [Google Scholar]
- 40.Mason R, Walter M, Trumbore M, Olmstead E, Mason P. Membrane antioxidant effects of the charged dihydropyridine calcium antagonist amlodipine. J Mol Cell Cardiol. 1999;31:275–281. doi: 10.1006/jmcc.1998.0867. [DOI] [PubMed] [Google Scholar]
- 41.Nayler W. The effect of amlodipine on hypertension-induced cardiac hypertrophy and reperfusion-induced calcium overload. J Cardiovasc Pharmacol. 1988;12:S41–S44. [PubMed] [Google Scholar]
- 42.Nayler WG. Experimental models to study the prevention of atherosclerosis by calcium antagonists. J Cardiovasc Pharmacol. 1995;26:S18–S24. [Google Scholar]
- 43.Ommen G, Goodman G, Thornquist M, Balmes J, Culien M. Effects of a combination of β-carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 44.Orth S, Nobiling R, Bönisch S, Ritz E. Inhibitory effect of calcium channel blockers on human mesangial cell growth: evidence for actions independent of L-type Ca2+ channels. Kidney Int. 1996;49:868–879. doi: 10.1038/ki.1996.120. [DOI] [PubMed] [Google Scholar]
- 45.Peterkofsky B, Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971;10:988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- 46.Phillips CL, Tajima S, Pinnell SR. Ascorbic acid and transforming growth factor-β1 increase collagen biosynthesis via different mechanisms: coordinate regulation of proα1(I) and proα1(III) collagens. Arch Biochem Biophys. 1992;295:397–403. doi: 10.1016/0003-9861(92)90533-3. [DOI] [PubMed] [Google Scholar]
- 47.Pitt B, Byington R, Furberg C, Hunninghake D, Mancini G, Miller M, Riley W. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. Circulation. 2000;102:1503–1510. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- 48.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 49.Roth M, Eickelberg O, Kohler E, Erne P, Block LH. Ca2+ channel blockers modulate metabolism of collagens within the extracellular matrix. Proc Natl Acad Sci USA. 1996;93:5478–5482. doi: 10.1073/pnas.93.11.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sajid M, Lele M, Stouffer G. Autocrine thrombospondin partially mediates TGF-β1-induced proliferation of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;279:H2159–H2165. doi: 10.1152/ajpheart.2000.279.5.H2159. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, O’Brien J, Fard A, Mannion J, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 52.Sima A, Stancu C, Constantinescu E, Ologeanu L, Simionescu M. The hyperlipemic hamster–a model for testing the anti-atherogenic effect of amlodipine. J Cell Mol Med. 2001;5:153–162. doi: 10.1111/j.1582-4934.2001.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon V, Moran M. SERCA activity is required for timely progression through G1/S. Cell Prolif. 2001;34:15–30. doi: 10.1046/j.1365-2184.2001.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stadius M, Gown A, Kernoff R, Collins C. Cell proliferation after balloon injury of iliac arteries in the cholesterol-fed New Zealand White rabbit. Arterioscler Thromb. 1994;14:727–733. doi: 10.1161/01.atv.14.5.727. [DOI] [PubMed] [Google Scholar]
- 55.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 56.Stepien O, Zhang Y, Zhu D, Marche P. Dual mechanism of action of amlodipine in human vascular smooth muscle cells. J Hypertens. 2002;20:95–102. doi: 10.1097/00004872-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Stepp DS, Tulenko TN. Alterations in basal and serotonin-stimulated Ca2+ movements and vasoconstriction in atherosclerotic aorta. Arterioscler Thromb. 1994;14:1854–1859. doi: 10.1161/01.atv.14.11.1854. [DOI] [PubMed] [Google Scholar]
- 58.Tliba O, Tliba S, Da Huang C, Hoffman R, DeLong P, Panettieri R, Amrani Y. Tumor necrosis factor alpha modulates airway smooth muscle function via the autocine action of interferon beta. J Biol Chem. 2001;278:50615–50623. doi: 10.1074/jbc.M303680200. [DOI] [PubMed] [Google Scholar]
- 59.Troup G, Wrenn S, Boesze-Battaglia K, Huang Y, Xie Y, Kirk T, Hanley F, Tulenko T. Membrane cholesterol and the formation of cholesterol domains in the pathogenesis of cardiovascular disease. Macromolecular Symposia: Chemistry and Physics of Colloids. In press. [Google Scholar]
- 60.Tulenko T, Sumner A, Chen M, Huang Y, Ferdinand F, Laury-Kleintop L. The smooth muscle cell membrane during atherogenesis: a potential target for amlodipine in atheroprotection. Am Heart J. 2001;141:1–10. doi: 10.1067/mhj.2001.109947. [DOI] [PubMed] [Google Scholar]
- 61.Tulenko TN, Chen M, Mason PE, Mason RP. Physical Effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J Lipid Res. 1998;39:947–956. [PubMed] [Google Scholar]
- 62.Turgan N, Habif S, Kabaroglu C, Mutaf I, Ozmen D, Bayindir O, Uysal A. Effects of the calcium channel blocker amlodipine on serum and aortic cholesterol, lipid peroxidation, antioxidant status and aortic histology in cholesterol-fed rabbits. J Biomed Sci. 2003;10:65–72. doi: 10.1007/BF02255999. [DOI] [PubMed] [Google Scholar]
- 63.Van der Meer BW, van Hoeven RP, van Blitterswijk WJ. Steady-state fluorescence polarization data in membranes. Resolution into physical parameters by an extended Perrin equation for restricted rotation of fluorophores. Biochim Biophys Acta. 1986;854:38–44. doi: 10.1016/0005-2736(86)90062-3. [DOI] [PubMed] [Google Scholar]
- 64.Waldron R, Short A, Meadows J, Ghosh T, Gill D. Endoplasmic reticulum calcium pump expression and control of cell growth. J Biol Chem. 1994;269:11927–11933. [PubMed] [Google Scholar]
- 64a.Walldius G, Erikson U, Olsson AG, Bergstrand L, Hadell K, Johansson K, Kaijser L, Lassvik C, Molgaard J, Nilsson S. The effect of probucol on femoral atherosclerosis: the Probucol Quantiative Regression Swedish Trial (PQRST) Am J Cardiol. 1994;74:875–883. doi: 10.1016/0002-9149(94)90579-7. [DOI] [PubMed] [Google Scholar]
- 65.Williams KJ, Tabas I. The response to retention hypothesis of early atherogenesis. Arterioscler Thromb. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamashita K, Tasaki H, Tsuda Y, Himeno E, Nakashima Y. Can aggressive lipid lowering using low density lipoprotein apheresis prevent restenosis after percutaneous transluminal coronary angioplasty in patients with normocholesterolemia. Therap Apheresis Dial. 1998;7:408–412. doi: 10.1111/j.1744-9987.1998.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 67.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation (HOPE) study investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 68.Zanchetti A, Bond M, Hennig M, Neiss A, Mancia G, Dal Palù C, Hansson L, Magnani B, Rahn KH, Reid J, Rodicio J, Safar M, Eckes L, Rizzini P. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation. 2002;106:2422–2427. doi: 10.1161/01.cir.0000039288.86470.dd. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Hintze T. Amlodipine releases nitric oxide from canine coronary microvessels: an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;97:576–580. doi: 10.1161/01.cir.97.6.576. [DOI] [PubMed] [Google Scholar]