Figure 1.
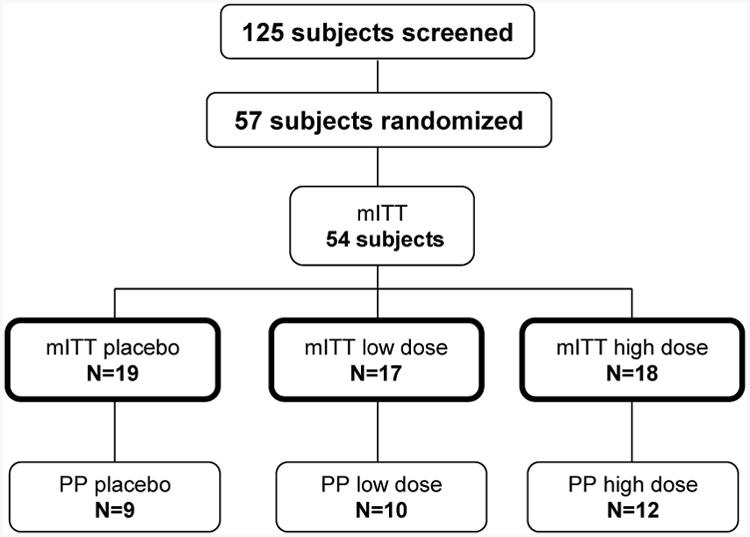
125 subjects were screened for eligibility into this study with randomization of 57 subjects (19 per treatment arm). The modified intent-to-treat (mITT) population was comprised of 54 subjects as 3 subjects never received a dose of study medication. The per-protocol (PP) population consisted of 31 subjects who had an overall treatment compliance 80-120% and had no substantive protocol deviations. (Subjects were excluded from the PP population if they had at least one of the following: noncompliant (n=6), negative IFN signature at baseline (n=7), receipt of prohibited medications at baseline (n=3), increase of immunosuppression during course of study (n=5), or, missing week 12 vitamin D (n=2) or IFN signature data (n=5)).
