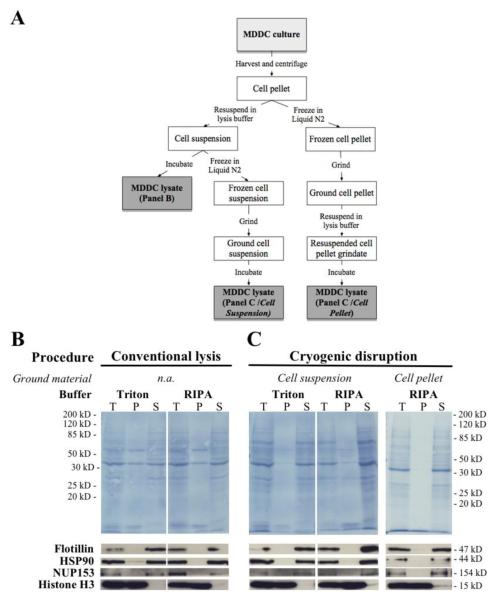
Figure 1.
Optimization of the procedure for extracting proteins from human monocyte-derived dendritic cells (MDDCs). The efficiency of protein extraction from MDDCs was assessed by comparing three different cellular disruption procedures in combination with two commonly used lysis buffers (as indicated and as described in Materials and Methods). (A) Schematic flowchart depicting the three cell disruption procedures used for this experiment. (B) After harvest, MDDCs were subjected to conventional lysis by incubating cells in the cold with the indicated lysis buffer. (C) Alternatively, MDDCs were subjected to cryogenic grinding either in suspension (Cell suspension) or as cell pellets (Cell pellet), prior to chemical lysis. In all cases, total cell lysates (T) were fractionated by centrifugal sedimentation to give rise to insoluble (P) and soluble (S) protein fractions. After SDS-PAGE and transfer, nitrocellulose membranes were stained with Amido Black to reveal the total protein content of each fraction. Subsequently, individual horizontal strips were subjected to immuno blot to reveal the fractionation pattern of each of the indicated cellular marker. Flotillin is a cell membrane protein associated with lipid rafts, HSP90 is a cytoplasmic and nucleoplasmic marker, Nup153 is a nucleoporin on the nuclear face of the nuclear membrane and Histone H3 is a chromatin protein found exclusively in the nucleus.