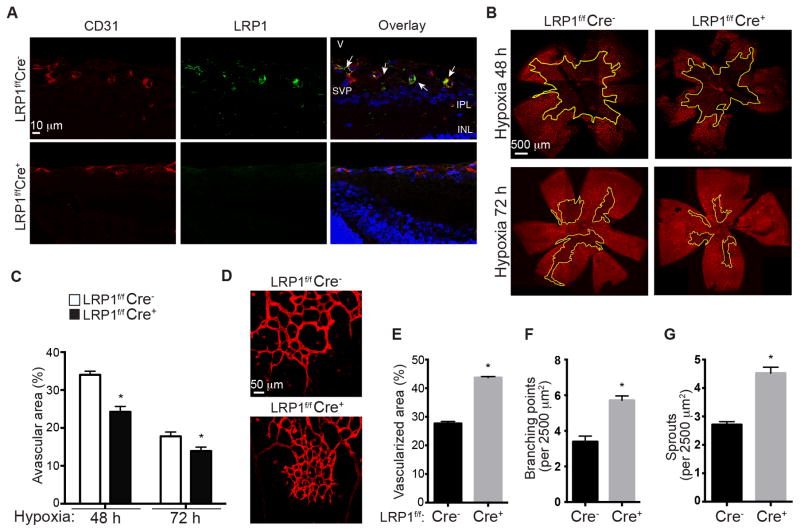
Figure 1. Intraretinal neovascularization increases in retinal endothelium lacking LRP1 during hypoxia.
A, Loss of LRP1 expression in endothelial cells in LRP1f/f;Cre+ mouse retina. Images are sagittal views of mouse retinas at postnatal day P15. Tissue sections were stained with LRP1 (8G1 Ab, green) and CD31 (an endothelial cell specific marker, red) antibodies. The test for the specificity of this 8G1 antibody is shown in Figure SII. INL, inner nuclear layer; IPL, inner plexiform layer; SVP, superficial vascular plexus; V, vitreous. B–C, Loss of LRP1 in endothelial cells increased intraretinal neovascularization following onset of hypoxia for 48 hours at P14 and 72 hours at P15. (B) Confocal images of retinal flat mounts from LRP1f/f;Cre+/− oxygen-induced retinopathy (OIR) mice were stained with iso-lectin. Avascularized (yellow outline) areas are shown as a percentage of the total area of the retinal superficial vasculature layer (C). *, P<0.05 via two-way ANOVA analysis followed by Bonferroni multiple comparison test, n≥4. D–G, Analysis of angiogenic parameters for the retinal neovascularization. D, An increase in neovascularization is observed at the leading edge of LRP1f/f;Cre+ retinas, compared to LRP1f/f;Cre− littermates. Confocal images of retinal flat mounts stained with iso-lectin from P15 LRP1f/f;Cre+/− oxygen-induced retinopathy mice were used for analysis. Vascularized area (E), branching pints (F) and vessel sprout number (G) were quantified within leading edge of neovascularization area. *, P<0.05 via unpaired Student’s t-test, n=4 for LRP1f/f;Cre− and n=3 for LRP1f/f;Cre+ mice.