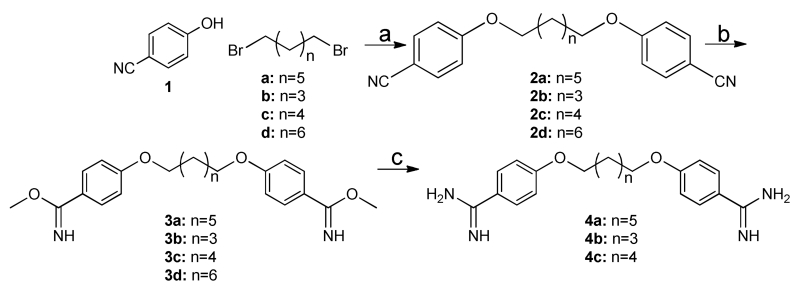
Scheme 1. Preparation of diamidines 4a-c.
Reagents and conditions: (a) CsCO3, acetone, reflux, 14 hrs.; (b) methanol:dioxane (1:1), HCl gas; (c) ethanolic ammonia, ammonium chloride, 80 °C, 32 hrs. Cyclic amidines (5a-d, 6a-d, and 7a-d) were synthesized according to literature with some modification (Scheme 2). Specifically, treatment of dibenzimidate with diaminoethane, diaminopropane and diaminobutane at room temperature in DMF accomplished cyclic amidine in satisfactory yield31-33. Increasing the chain length of the diamine results in open chain products (8 and 9a, 9b), which were useful for testing aliphatic chain derivatives of varying lengths (Scheme 2).