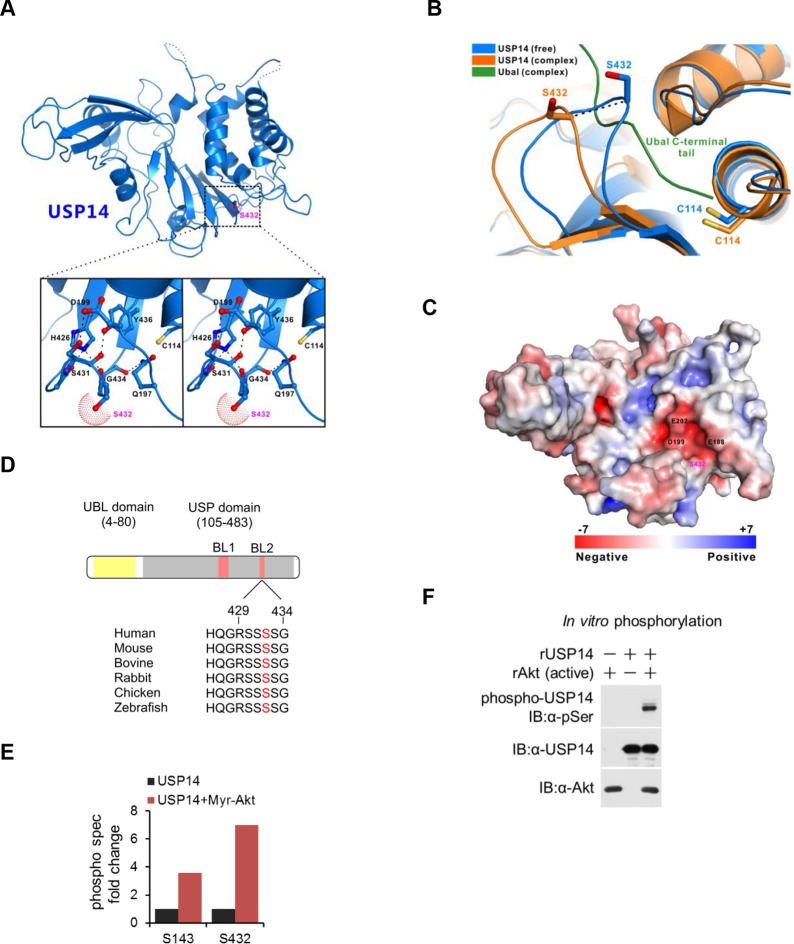
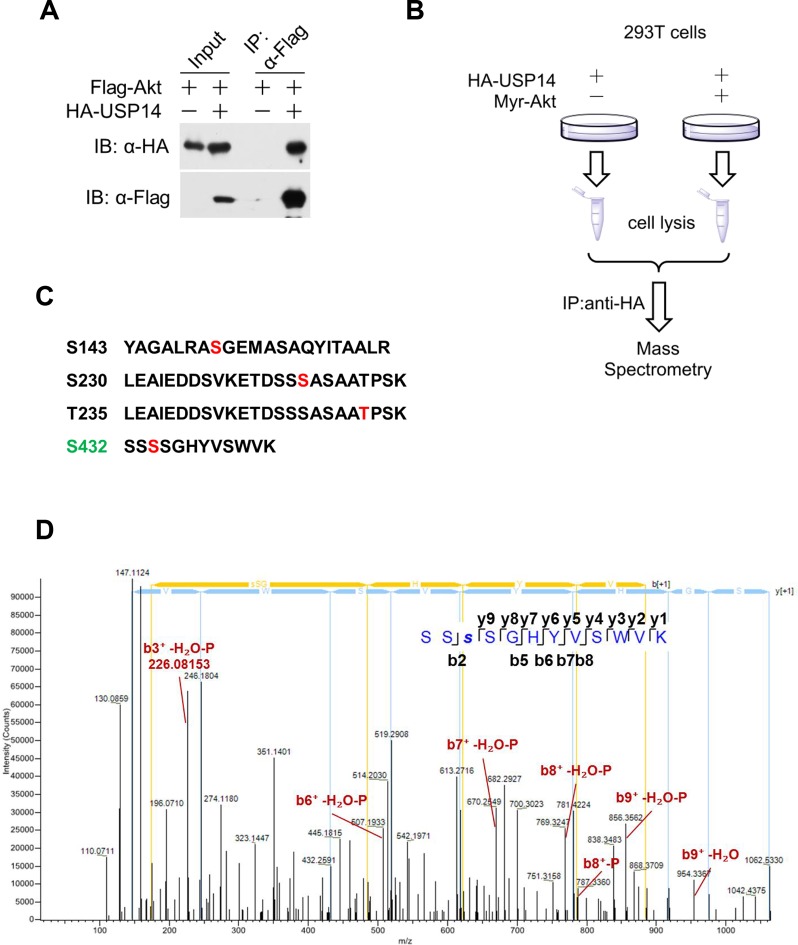
Figure 1. Structural basis of ubiquitin-specific protease-14 (USP14) activation by phosphorylation of Ser432.
(A) Detailed view of blocking loop 2 (BL2), which occludes the active site of USP14 (PDB access code 2AYN). The BL2 loop, which contains Ser432, is shown in stick model, in the apo form. (B) Combined ribbon representation and stick model showing a comparison of the conformations of the BL2 loop contained in the apo form (blue, PDB access code 2AYN) and in the USP14- Ub-aldehyde (Ubal) adduct (orange, PDB access code 2AYO). In this drawing, the Ser432 and Cys114 residues are shown in stick model, and the bound Ubal (a ubiquitin derivative in which the C-terminal carboxylate is replaced by an aldehyde) in the complex is drawn in green. (C) A surface charge potential representation (contoured at ± 7 kT/eV; blue/red) of USP14 (PDB accession 2AYN) showing that the S432 residue is very close to a highly negatively charged patch mainly formed by the acidic E188, D199, and E202 residues. When S432 is phosphorylated, the negatively charged phosphate group may induce a repulsive force, thereby relieving inhibition of the catalytic activity of USP14. (D) USP14 domain organization and sequence alignment of the Akt phosphorylation site within USP14 orthologs from different species. Two BLs (BL1 and BL2) covering the USP14 active site are shown. The Akt phosphorylation site in USP14 from different species as predicted by Scansite. (E) S432 is the major phosphorylation site in USP14. HEK293T cells were treated as in Figure 1—figure supplement 1B, followed by electrospray ionization mass spectrometry (ESI-MS) analysis. Spectral counts were determined by ESI-MS. (F) Akt phosphorylates USP14 in vitro. Bacterially expressed and purified USP14 was incubated with active Akt in the presence of ATP. Reaction products were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and phosphorylated species were detected by a phospho-Ser antibody.