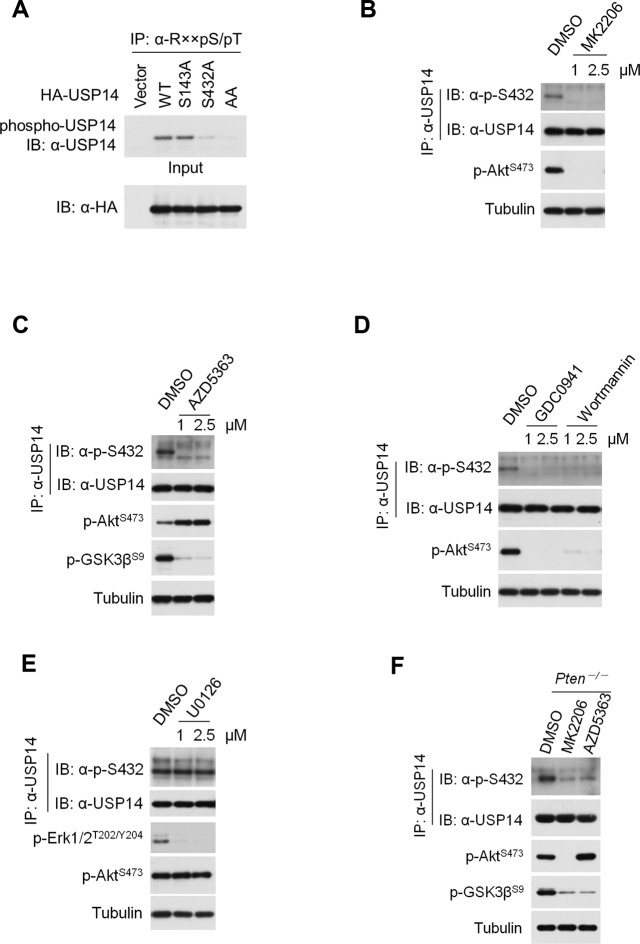
Figure 2. Ubiquitin-specific protease-14 (USP14) is phosphorylated at Ser432 by activated Akt.
(A) In vitro phosphorylation of USP14 at S432 by Akt. Bacterially expressed and purified wild type USP14 or AA mutant incubated with active Akt in the presence of ATP. Reaction products were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and phosphorylation was detected by the phospho-Ser antibody. (B) Akt phosphorylates USP14 at S432 in vivo. Western blot analysis of whole cell lysate and immunoprecipitates derived from HEK293T cells transfected with wild type USP14, USP14 S143A, USP14 S432A, and USP14 S143A/S432A (AA) constructs using the phospho-Ser antibody. L.E., long exposure. (C) Immunoprecipitation (IP) and IB analysis of HEK293T cells transfected with HA-USP14 and Myr-Akt and preincubated with or without λ-phosphatase as indicated. (D) Inhibition of Akt decreased exogenous USP14 phosphorylation. HEK293T cells were transfected with Myc-USP14 for 20 hr then treated with 1 μM MK2206 or deprived of serum for another 4 hr before harvest. (E) In vitro kinase assay to detect Akt phosphorylation of USP14 by phospho-Ser432-specific antibody and phos-tag-containing gels. Bacterially expressed and purified wild type USP14 or S432A mutant was incubated with active Akt in the presence of ATP. The reaction products were resolved by SDS-PAGE, and USP14 phosphorylation was detected using an antibody that specifically recognizes Ser432 phosphorylation of USP14 or determined by differential migration on phos-tag gels. (F) In vivo detection of endogenous USP14 Ser432 phosphorylation by anti-p-Ser432-specific antibody. Western blot analysis of immunoprecipitates derived from H4 cells transfected with or without Myr-Akt plasmids using the anti-p-Ser432-specific antibody. (G, H) Phosphorylation of endogenous USP14 S432 upon stimulation with insulin-like growth factor (IGF-1) or epidermal growth factor (EGF). HEK293T cells were serum-starved and pretreated with Akt inhibitor MK2206 (1 μM) for 30 min before stimulation with IGF-1 (100 ng/mL) for 30 min (G) or EGF (100 ng/mL) for 1 hr (H). The cell lysates were immunoprecipitated with USP14 antibody and western-blotted with anti-p-S432 antibody. (I) Phosphorylation of endogenous USP14 S432 in Pten knockout cells with high activity of Akt. Lysates from mouse embryonic fibroblasts (MEFs) with indicated genotypes were immunoprecipitated with USP14 antibody and then Western blotted with p-S432 antibody. The differential migration of phospho-USP14 on phos-tag-containing gels was determined as shown in the bottom panel.