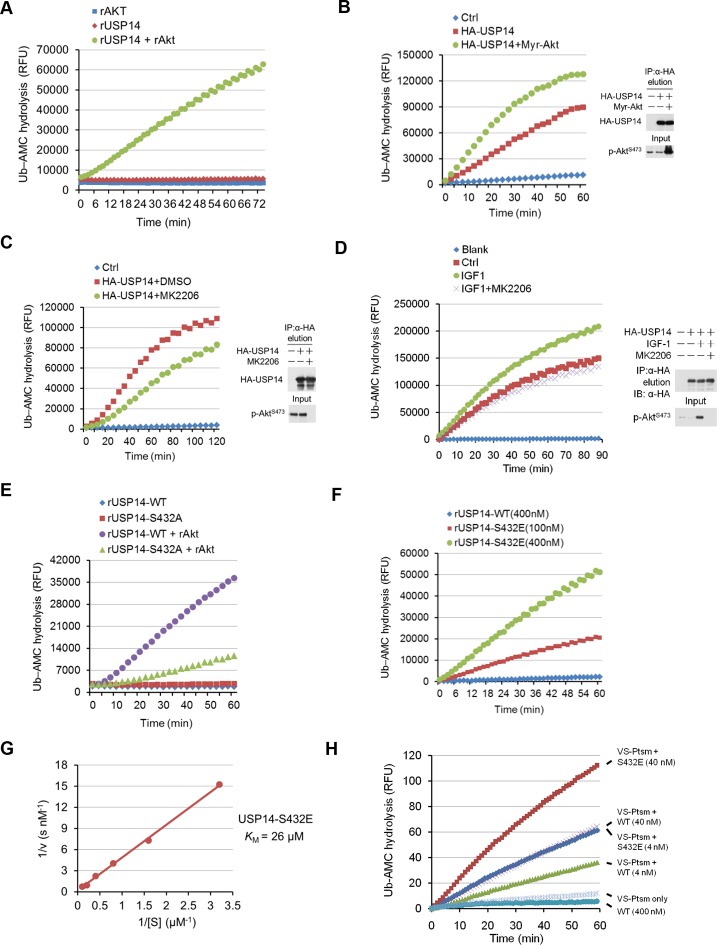
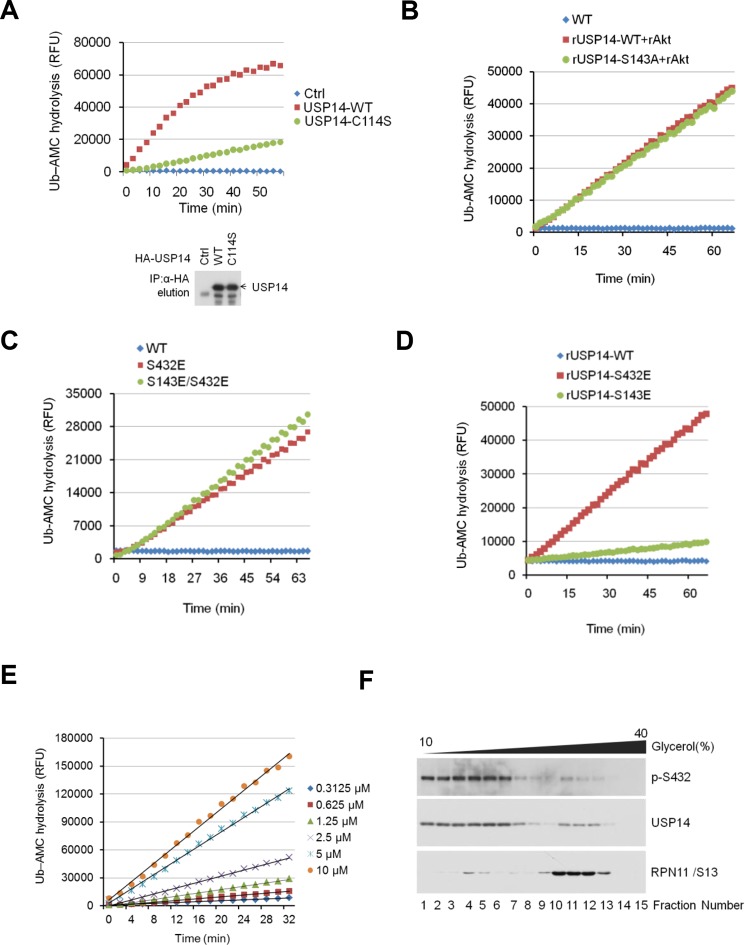
Figure 3. Phosphorylation of ubiquitin-specific protease-14 (USP14) by Akt activates USP14 DUB activity.
(A) Akt activates USP14 DUB activity in vitro. USP14 protein (1 μg) was incubated with or without active Akt (1 μg) in kinase assay buffer in a total volume of 50 μL for 1 hr at 30°C, then the reaction mixtures were subjected to Ub-AMC assay. RFU, relative fluorescence units. (B, C) Akt activates USP14 in cells. USP14 was immunoprecipitated from HEK293T cells coexpressed with activated Akt (B) or treated with 10 μM MK2206 for 4 hr (C) and then eluted with HA-peptide following Ub-AMC hydrolysis assay. (D) Activation of USP14 by stimulating cells with IGF-1. HEK293T cells were serum-starved and pretreated with or without Akt inhibitor MK2206 (1 μM) for 30 min before stimulation with IGF-1 (100 ng/mL) for 30 min. USP14 was then immunoprecipitated and eluted with HA-peptide. The activity of USP14 was determined using Ub-AMC hydrolysis assay. (E) USP14 activation by Akt is blocked by S432A mutation. Ub-AMC hydrolysis assay of wildde type USP14 or S432A mutant in the presence or absence of active Akt. (F) Ub-AMC hydrolysis assay of bacterially expressed and purified wild type USP14 or S432E mutant. (G) Lineweaver–Burk analysis of USP14 S432E, obtained by measuring the initial rates at varying Ub-AMC concentrations (see Figure 3—figure supplement 2E for reference). (H) The activity of phospho-mimetic USP14 mutant can be further stimulated by the presence of proteasome. Ub-AMC hydrolysis assay of wild type USP14 or S432E mutant in the presence or absence of Ub-VS-treated human proteasome (VS-proteasome (see Lee et al., 2010); 1 nM). Ptsm, 26S proteasome.