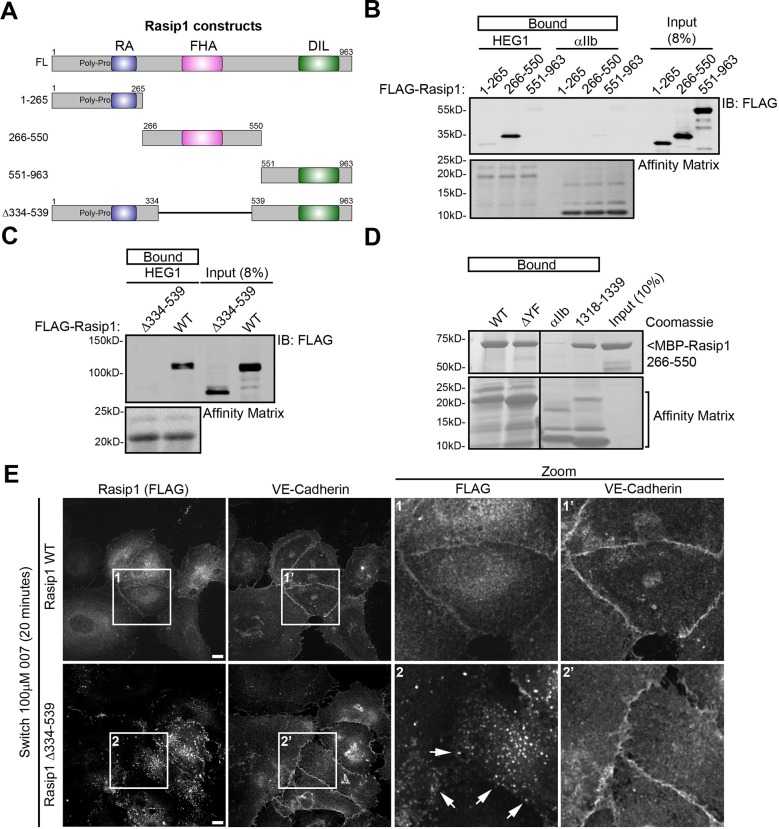
Figure 5. Rasip1 central domain interacts with HEG1 cytoplasmic tail.
(A) Schematic representation of Rasip1 constructs. (B) HEK293T cells were transfected with FLAG-tagged Rasip1 1-265, 266-550, or 551-963. Western blot analysis shows that the HEG1 cytoplasmic tail peptide preferentially bound to FLAG-Rasip1 266-550, which contains an FHA domain. αIIb cytoplasmic tail was used as a control. Affinity Matrix was visualized by Ponceau staining. Data are representative of at least 3 independent experiments. (C) HEK293T cells were transfected with FLAG-tagged wild-type Rasip1 (WT) or Rasip1(∆334-539), which lacks the FHA domain. Western blot analysis shows that, in contrast to Rasip1 wild-type, the HEG1 cytoplasmic tail did not interact with Rasip1(∆334-539). Affinity Matrix was visualized by Ponceau staining. Data are representative of at least 3 independent experiments. (D) Wild-type (WT) HEG1 cytoplasmic tail peptide, ∆YF, and HEG1 1318-1339, but not αIIb cytoplasmic tail, directly bound to recombinant MBP-Rasip1 266-550 fusion protein. Coomassie blue-stained SDS-PAGE gel is representative of 3 independent experiments. All lanes were from the same gel. (E) FLAG-Rasip1 intracellular distribution was analyzed by Spinning Disk Confocal Microscopy (SDCM) in Human Umbilical Vein Endothelial Cells (HUVEC) expressing FLAG-tagged wild-type (WT) Rasip1 or Rasip1(∆334-539) expressed by lentiviral infection. Cells were treated with DMEM (5% FBS, 4 mM EGTA) to remove Calcium and disrupt adherens junctions. Subsequently, cells were incubated with DMEM containing 8-pCPT-2-O-Me-cAMP-AM ('007', 100 µM) and Calcium (2 mM) for 20 minutes to mimic junction formation/stabilization. Under these conditions, Rasip1 WT localized to cell-cell contacts in. In contrast, Rasip1(∆334-539) failed to localize to junctions albeit Rasip1-positive vesicular structures could be found in the vicinity of cell-cell contacts (indicated by the arrows). Higher magnification images of the boxed area are included. Representative images of 3 independent experiments are shown. Scale bars, 10 µm.