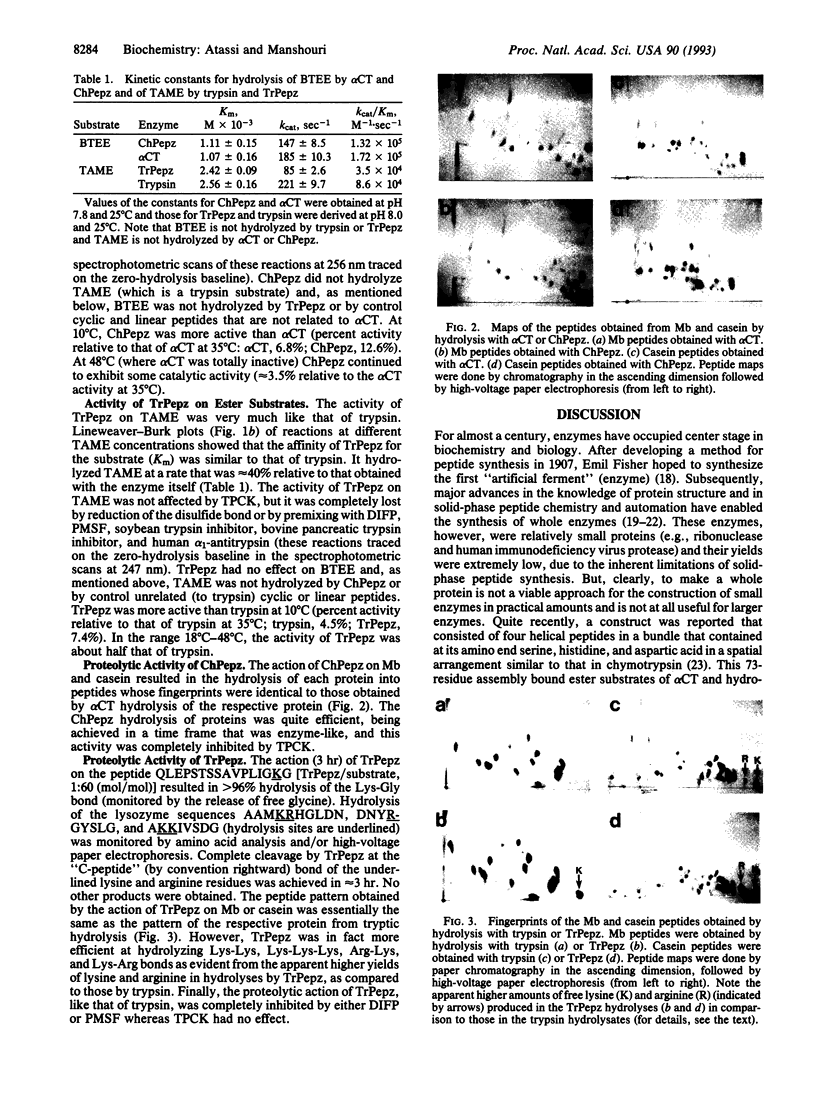
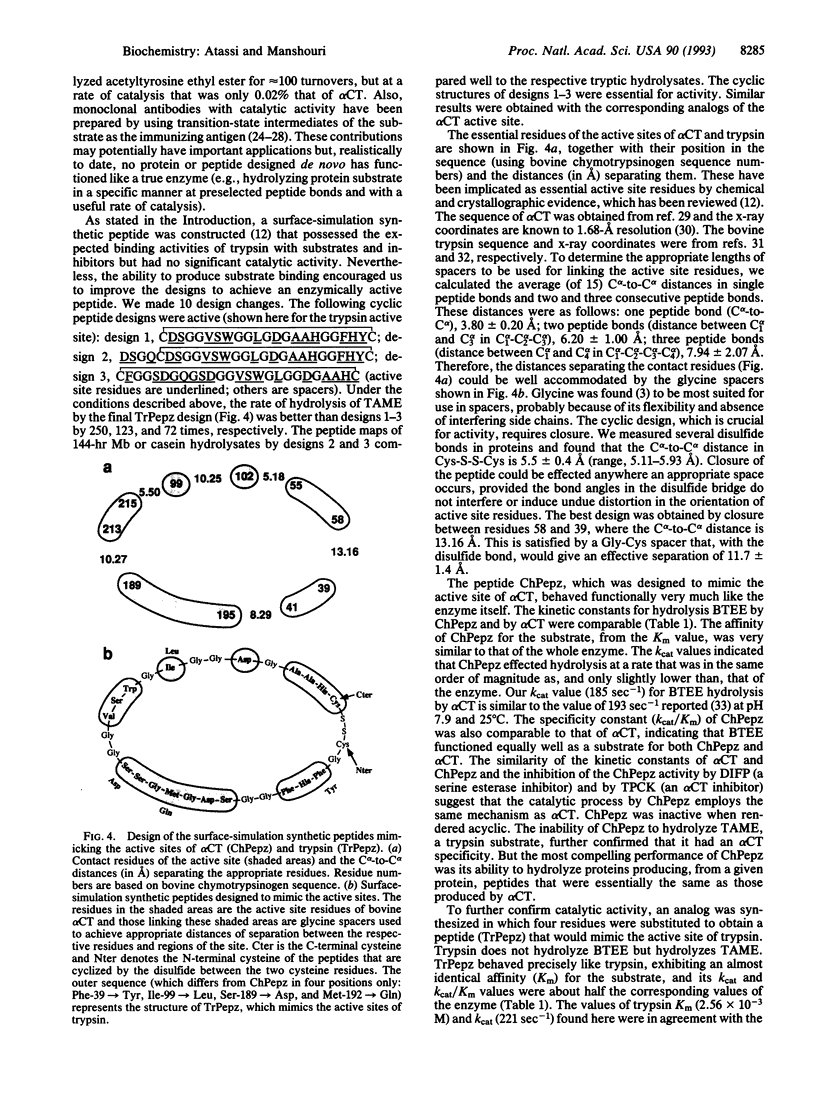
Abstract
Two 29-residue peptides were prepared, one of which (ChPepz) was designed by surface-simulation synthesis to mimic the active site of alpha-chymotrypsin, and the other (TrPepz), which contained four substitutions relative to ChPepz, was fashioned after the active site of trypsin. Each peptide was cyclized by a disulfide bond. The ChPepz monomer effected hydrolysis of the ester group in N-benzoyl-L-tyrosine ethyl ester, an alpha-chymotrypsin substrate, with Km and kcat values that were comparable to those of alpha-chymotrypsin. ChPepz was completely inactivated by diisopropyl fluorophosphate (DIFP), L-1-p-tosylamino-2-phenylethyl chloromethyl ketone (TPCK), or reduction of the disulfide bond. It had no catalytic activity on N-tosyl-L-arginine methyl ester, a trypsin substrate. On the other hand, TrPepz, which had no effect on N-benzoyl-L-tyrosine ethyl ester, hydrolyzed N-tosyl-L-arginine methyl ester with a Km value that was essentially identical to that of trypsin, but its kcat value was almost half that of trypsin. TrPepz was fully inactivated by reduction of the disulfide bond, by DIFP, or by phenylmethylsulfonyl fluoride but not by TPCK. It was also completely inhibited by soybean trypsin inhibitor, bovine pancreatic trypsin inhibitor, and human alpha 1-antitrypsin. ChPepz and TrPepz hydrolyzed proteins (myoglobin and casein) to give panels of peptides that were similar to those of the same protein obtained with the respective enzyme. However, TrPepz was more efficient than trypsin at hydrolyzing the C bonds of two or more consecutive lysine and/or arginine residues. Like its esterase activity, the proteolytic activity of ChPepz was inhibited by either DIFP or TPCK whereas that of TrPepz was inhibited by either DIFP or phenylmethylsulfonyl fluoride but not by TPCK. Finally, ChPepz and TrPepz were each more active at low temperature than the respective enzyme. This ability to construct fully functional peptide enzymes (pepzymes) of chosen specificities should find many practical applications.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATASSI M. Z. PROPERTIES OF COMPONENTS OF MYOGLOBIN OF THE SPERM WHALE. Nature. 1964 May 2;202:496–498. doi: 10.1038/202496a0. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L. Boundary refinement of the lysozyme antigenic site around the disulphide bond 6-127 (site 1) by 'surface-simulation' synthesis. Biochem J. 1978 May 1;171(2):419–427. doi: 10.1042/bj1710419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L., Pai R. C. Enzymic and immunochemical properties of lysozyme. XVI. A novel synthetic approach to an antigenic reactive site by direct linkage of the relevant conformationally adjacent residues constituting the site. Biochim Biophys Acta. 1976 Apr 14;427(2):745–751. doi: 10.1016/0005-2795(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Lee C. L. The precise and entire antigenic structure of native lysozyme. Biochem J. 1978 May 1;171(2):429–434. doi: 10.1042/bj1710429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Manshouri T., Sakata S. Localization and synthesis of the hormone-binding regions of the human thyrotropin receptor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3613–3617. doi: 10.1073/pnas.88.9.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Precise determination of the entire antigenic structure of lysozyme: molecular features of protein antigenic structures and potential of "surface-simulation" synthesis--a powerful new concept for protein binding sites. Immunochemistry. 1978 Dec;15(12):909–936. doi: 10.1016/0161-5890(78)90126-8. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Saplin B. J. Immunochemistry of sperm whale myoglobin. I. The specific interaction of some tryptic peptides and of peptides containing all the reactive regions of the antigen. Biochemistry. 1968 Feb;7(2):688–698. doi: 10.1021/bi00842a026. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Surface-simulation synthesis of the substrate-binding site of an enzyme. Demonstration with trypsin. Biochem J. 1985 Mar 1;226(2):477–485. doi: 10.1042/bj2260477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Zablocki W. Can an antibody-combining site be mimicked synthetically? The possible surface simulation synthesis of two antibody-combining sites complementary to two antigenic sites of lysozyme. J Biol Chem. 1977 Dec 25;252(24):8784–8787. [PubMed] [Google Scholar]
- Baldwin E., Schultz P. G. Generation of a catalytic antibody by site-directed mutagenesis. Science. 1989 Sep 8;245(4922):1104–1107. doi: 10.1126/science.2672338. [DOI] [PubMed] [Google Scholar]
- Gutte B., Merrifield R. B. The synthesis of ribonuclease A. J Biol Chem. 1971 Mar 25;246(6):1922–1941. [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Hahn K. W., Klis W. A., Stewart J. M. Design and synthesis of a peptide having chymotrypsin-like esterase activity. Science. 1990 Jun 22;248(4962):1544–1547. doi: 10.1126/science.2360048. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. R., Prudent J. R., Kochersperger L., Yonkovich S., Schultz P. G. An efficient antibody-catalyzed aminoacylation reaction. Science. 1992 Apr 17;256(5055):365–367. doi: 10.1126/science.256.5055.365. [DOI] [PubMed] [Google Scholar]
- Janda K. D., Benkovic S. J., Lerner R. A. Catalytic antibodies with lipase activity and R or S substrate selectivity. Science. 1989 Apr 28;244(4903):437–440. doi: 10.1126/science.2717936. [DOI] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Antibody combining sites can be mimicked synthetically. Surface-simulation synthesis of the phosphorylcholine-combining site of myeloma protein M-603. Biochem J. 1980 Jun 1;187(3):661–666. doi: 10.1042/bj1870661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAND L., RULE N. G. Inhibition of proteolytic enzymes by decarboxylated amino-acid derivatives. Effect of toluenesulphonyl(tosyl)agmatine (4-toluenesulphonamidobutyl-guanidine) on thrombin trypsin. Nature. 1961 May 20;190:722–723. doi: 10.1038/190722a0. [DOI] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Delineation of the third antigenic site of lysozyme by application of a novel 'surface-simulation' synthetic approach directly linking the conformationally adjacent residues forming the site. Biochem J. 1976 Oct 1;159(1):89–93. doi: 10.1042/bj1590089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Accurate definition of the antigenic site around the disulphide bridge 30-115 (site 3) by 'surface-simulation' synthesis. Biochem J. 1977 Dec 1;167(3):571–581. doi: 10.1042/bj1670571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. XIX. Definition of the direction and conformational restrictions of the antigenic site around the disulfide bonds 64-80 and 76-94 (site 2) by 'surface-simulation' synthesis. Biochim Biophys Acta. 1977 Dec 20;495(2):354–368. doi: 10.1016/0005-2795(77)90391-9. [DOI] [PubMed] [Google Scholar]
- MARTIN C. J., GOLUBOW J., AXELROD A. E. The hydrolysis of carbobenzoxy-L-tyrosine p-nitrophenyl ester by various enzymes. J Biol Chem. 1959 Jul;234(7):1718–1725. [PubMed] [Google Scholar]
- Meloun B., Kluh I., Kostka V., Morávek L., Prusík Z., Vanecek J., Keil B., Sorm F. Covalent structure of bovine chymotrypsinogen A. Biochim Biophys Acta. 1966 Dec 28;130(2):543–546. doi: 10.1016/0304-4165(66)90258-3. [DOI] [PubMed] [Google Scholar]
- Mikes O., Holeysovský V., Tomásek V., Sorm F. Covalent structure of bovine trypsinogen. The position of the remaining amides. Biochem Biophys Res Commun. 1966 Aug 12;24(3):346–352. doi: 10.1016/0006-291x(66)90162-8. [DOI] [PubMed] [Google Scholar]
- Miller M., Schneider J., Sathyanarayana B. K., Toth M. V., Marshall G. R., Clawson L., Selk L., Kent S. B., Wlodawer A. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science. 1989 Dec 1;246(4934):1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- Milton R. C., Milton S. C., Kent S. B. Total chemical synthesis of a D-enzyme: the enantiomers of HIV-1 protease show reciprocal chiral substrate specificity [corrected]. Science. 1992 Jun 5;256(5062):1445–1448. doi: 10.1126/science.1604320. [DOI] [PubMed] [Google Scholar]
- Pollack S. J., Jacobs J. W., Schultz P. G. Selective chemical catalysis by an antibody. Science. 1986 Dec 19;234(4783):1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kent S. B. Enzymatic activity of a synthetic 99 residue protein corresponding to the putative HIV-1 protease. Cell. 1988 Jul 29;54(3):363–368. doi: 10.1016/0092-8674(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Shokat K. M., Schultz P. G. Catalytic antibodies. Methods Enzymol. 1991;203:327–351. doi: 10.1016/0076-6879(91)03019-d. [DOI] [PubMed] [Google Scholar]
- Tsukada H., Blow D. M. Structure of alpha-chymotrypsin refined at 1.68 A resolution. J Mol Biol. 1985 Aug 20;184(4):703–711. doi: 10.1016/0022-2836(85)90314-6. [DOI] [PubMed] [Google Scholar]
- Twining S. S., Atassi M. Z. Antibody-combining sites can be mimicked synthetically. Surface-simulation synthesis of the immunoglobulin new combining site to the gamma-hydroxyl derivative of vitamin K1. J Biol Chem. 1978 Aug 10;253(15):5259–5262. [PubMed] [Google Scholar]