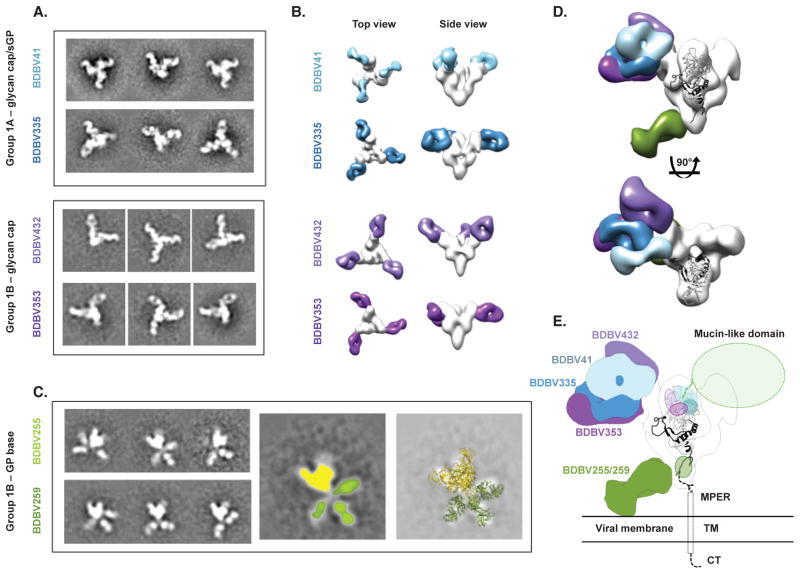
Figure 4. BDBV-neutralizing antibodies bind to the glycan cap or base region of GP.
(A) Shown are negative-stain electron microscopy reference-free 2D class averages of Group 1A antibodies that bind both the glycan cap of GP and sGP, and Group 1B antibodies that bind the glycan cap of GP but not sGP. BDBV GP or GPΔmuc was used to generate complexes. (B) 3D reconstructions of glycan cap binders from Groups 1A and 1B reveal that these antibodies bind the glycan cap at overlapping but distinct epitopes. Top (left) and side (right) views of the complexes are shown. (C) Reference free 2D class averages of Group 1B antibodies (left) reveals that these antibodies bind an epitope below the base of GP that is flexible. In the middle image, GP is colored yellow and each Fab colored green. The right-hand panel illustrates a superimposition of crystal structures of SUDV GPΔmuc (PDB 3VEO) and Fabs (PDB 3CSY) to demonstrate how Fabs may bind to GP. (D) The composite model delineates the epitopes of the glycan cap mAbs in Group 1A or 1B. Side (above) and top (below) views are shown. (E) Docking a crystal structure of SUDV GPΔmuc (PDB 3VEO) (Bale et al., 2012), which contains a more complete model of the glycan cap region targeted by Group 1A/B mAbs, reveals how Group 1A/B mAbs target a broad region in the GP1 centered on the glycan cap, near the beginning of the mucin-like domains. Group 1B mAbs that target the base likely bind to a loop near the membrane proximal external region (MPER) that is flexible and has not yet been resolved at high resolution. TM = transmembrane region; CT = cytoplasmic tail. See also Figure S4.