Abstract
The purpose of this study was to explore long-term complications in recipients of deceased donor liver transplant (DDLT) and living donor liver transplant (LDLT) in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL). We analyzed 471 DDLTs and 565 LDLTs from 1998 to 2010 followed up to 10 years for 36 categories of complications. Probabilities of complications and their resolution were estimated using Kaplan-Meier and predictors were tested in Cox models. Median follow-up for DDLT and LDLT was 4.19 and 4.80 years, respectively. DDLT recipients were more likely to have hepatocellular carcinoma (HCC) and higher disease severity, including Model for End-stage Liver Disease (MELD) score. Complications occurring with higher probability in LDLT included biliary-related complications and hepatic artery thrombosis (HAT). In DDLT, ascites, intra-abdominal bleeding, cardiac complications, and pulmonary edema were significantly more probable. Development of chronic kidney disease (CKD) stage 4 or 5 was less likely in LDLT recipients (HR 0.41, p=0.02). DDLT and LDLT had similar risk of grade 4 complications (HR=0.89, p=0.60), adjusted for other risk factors. Once a complication occurred, the time to resolution did not differ between LDLT and DDLT. Future efforts should be directed towards reducing the occurrence of complications after liver transplantation.
Keywords: Liver transplantation, post-operative complications, living donor
Introduction
Living donor liver transplantation (LDLT) was developed to enable the use of liver replacement therapy in areas where deceased donor liver allografts were in far too short supply. In the United States, LDLT has been shown to significantly reduce waiting list mortality (1) and is associated with improved 5-year survival (2). Application in the United States has been limited by both concerns about donor safety, as well as the increased technical difficulty associated with LDLT. The Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL), a consortium of nine US centers with significant experience in adult-to-adult living donor liver transplantation, reported that Clavien grade IV complications (defined as re-transplantation or death) were more common in the early experience of a center (cases 1-20) (3), with an additional increased risk of vascular complications (3). The risk of biliary complications was also significantly higher for both biliary leaks and biliary strictures versus patients who underwent deceased donor liver transplantation (DDLT) (3). High rates of biliary complications have been similarly observed in single center studies of LDLT (4, 5). LDLT recipients have also been shown to have higher rates of post-transplant hospitalization (6, 7).
There are limited comparative data on post-transplant complications after LDLT and DDLT in the US, because publicly available national data contain limited detail on specific complications. In this study, we analyzed 471 DDLT and 565 LDLT transplants from the A2ALL consortium to better understand the complications and morbidity associated with DDLT and LDLT over a 12-year period. We show the probability and risk associated with each procedure of developing the most common complications and the time course and likelihood of their resolution.
Methods
Data Collection
Potential living donor transplant recipients who had a donor evaluated between January 1, 1998 and August 31, 2009 were enrolled at each of the nine A2ALL centers beginning in the third quarter of 2004 and followed through August 31, 2010. These potential recipients may have ultimately received an LDLT, a DDLT, or neither. Clinical data, including laboratory data, hospitalizations, and complications, were collected based on a common protocol, with study visits occurring at post-donation weeks 1 and 2, months 1, 3, and 12, and annually thereafter. Data collected on post-transplant complications included whether the transplant was an LDLT or DDLT, dates of onset and resolution, as well as information required for grading of severity using the Clavien classification scheme (8–10). Subjects could enroll either before or after transplant, and information prior to enrollment was collected via chart review. Clinical and laboratory data, including patient and graft survival, deceased donor characteristics, and serial creatinine values were supplemented using data from the Scientific Registry of Transplant Recipients (SRTR) when they were not available in the A2ALL clinical database. The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
The A2ALL study includes 1,208 successful transplant recipients, as well as 14 whose transplant was aborted for recipient reasons (total n=1222). LDLT recipients whose transplants (whether complete or aborted) were among the first 20 LDLT cases at their center were excluded (n=175, including 2 aborted donations) because this learning curve period has been previously shown to be associated with a higher probability of complications (3); DDLT recipients during the early LDLT period at each center were included. Subjects who could not be linked to the SRTR were also excluded due to resulting data limitations; SRTR was the major source for data on DDLT donors. A total of 1,036 patients proceeded to the operating room with the intention of receiving an LDLT (n=565) or DDLT (n=471) and were included for analysis. Transplant procedures aborted for recipient reasons (1 LDLT, 11 DDLT) were included in Table 1 but excluded from subsequent analyses. Of the 1,036 patients in this report, 422 were previously included in Freise et al.(3) The current study includes 1,289 person-years of new follow-up in addition to the 820 person-years reported in the previous publication.
Table 1.
Recipient Characteristics at Transplant
| DDLT (n=471) | LDLT (n=565)β | ||||
|---|---|---|---|---|---|
| N or Mean | % or SD | N or Mean | % or SD | P-valueδ | |
| Age | 52.2 | 10.4 | 51.0 | 10.9 | 0.09 |
| Gender | 0.08 | ||||
| Male | 285 | 61 | 311 | 55 | |
| Female | 186 | 39 | 254 | 45 | |
| Ethnicity | 0.56 | ||||
| Hispanic/Latino | 89 | 19 | 99 | 18 | |
| Non-Hispanic/Non-Latino | 381 | 81 | 466 | 82 | |
| Missing | 1 | <1 | 0 | 0 | |
| Race | 0.01 | ||||
| White | 397 | 84 | 514 | 91 | |
| African American | 32 | 7 | 21 | 4 | |
| Asian | 18 | 4 | 12 | 2 | |
| Other | 24 | 5 | 18 | 3 | |
| Body mass index (kg/m2) 1 | 26.7 | 4.9 | 26.5 | 5.2 | 0.55 |
| Recipient Diagnosis (multiple diagnoses possible) | |||||
| HCV | 214 | 45 | 225 | 40 | 0.07 |
| HCC | 103 | 22 | 70 | 12 | <0.0001 |
| Alcohol | 54 | 11 | 57 | 10 | 0.48 |
| Cholestatic Liver Disease | 93 | 20 | 142 | 25 | 0.04 |
| Noncholestatic cirrhosis other than HCV/Alcohol | 95 | 20 | 114 | 20 | 1.00 |
| Acute hepatic necrosis | 19 | 4 | 17 | 3 | 0.37 |
| Metabolic Disease | 19 | 4 | 15 | 3 | 0.21 |
| Biliary Atresia | 0 | 0 | 7 | 1 | 0.02 |
| Malignancy other than HCC | 9 | 2 | 18 | 3 | 0.20 |
| Other | 107 | 23 | 116 | 21 | 0.39 |
| MELD1 | 20.3 | 8.9 | 15.2 | 5.7 | <0.0001 |
| 6–15 | 159 | 34 | 320 | 57 | |
| 16–20 | 107 | 23 | 160 | 28 | |
| 21–30 | 120 | 25 | 68 | 12 | |
| 31–40 | 70 | 15 | 10 | 2 | |
| Missing | 15 | 3 | 7 | 1 | |
| Medical Condition at Transplant | <0.0001 | ||||
| Patient in ICU | 50 | 11 | 8 | 1 | |
| Hospitalized, not in ICU | 70 | 15 | 34 | 6 | |
| Not Hospitalized | 344 | 73 | 522 | 92 | |
| Missing | 7 | 1 | 1 | <1 | |
| Severity | |||||
| Ventilator | 28 | 6 | 8 | 1 | 0.0001 |
| Ascites | 282 | 60 | 255 | 45 | <0.0001 |
| Dialysis | 25 | 5 | 4 | 1 | <0.0001 |
| Intraoperative3,4 | |||||
| Cold Ischemia Time (min) 2 | 446.1 | 181.6 | 66.4 | 138.3 | <0.0001 |
| Duration of recipient operation (min) 2 | 369.2 | 133.7 | 476.1 | 121.8 | <0.0001 |
Excludes LDLT case number ≤ 20.
P-values comparing LDLT versus DDLT for continuous variables are based on t-tests, and for categorical variables are based on the chi-square or Fisher’s exact tests.
Values missing less than 5% for these variables.
Values missing less than 11% for these variables.
N=11 aborted DDLT transplants excluded.
N=1 aborted LDLT transplant excluded.
Human Subjects Protection
This study was approved by the Institutional Review Boards of the University of Michigan Data Coordinating Center and each of the nine A2ALL centers. University of Michigan’s IRBMED is 2002-0484 (HUM00045813). Colorado is 03-1115; Columbia is AAAA2671; Northwestern is CR2_STU00008840; Penn is 801069; UNC is 03-1484; UCLA is 02-11-006-21; UCSF is 10-03052; UVA is 11066; and VCU is 3560.
Statistical Methods
Descriptive statistics for LDLT and DDLT recipients are shown as means, standard deviations, frequencies, and percentages. Comparisons between LDLT and DDLT recipients were made using t-tests for continuous variables and chi-square or Fisher’s exact test for categorical variables. Unadjusted probabilities of the first occurrence of specific complications by time since transplant for the first year post-transplant and over all follow-up were estimated using the Kaplan-Meier method; comparisons between LDLT and DDLT were made using the log-rank test. Similar methods were used to estimate the probability of resolution of selected complications for all occurrences of each complication. Specific complications were categorized as being significantly higher in either LDLT or DDLT or not significantly different based on the unadjusted log-rank p-value over all follow-up.
Data on recipient BMI, MELD, and components of the donor risk index (DRI) for DDLT recipients including donor age, race, height, cause of death, cold ischemia time, liver type (split vs. not), donation after cardiac death, liver origin, and medical condition at transplant were missing in less than 5% while cold ischemia time and duration of operation were missing in around 10%. Missing covariate data were imputed five times using the multiple imputation algorithm in IVEware software to generate five complete datasets. Each dataset was analyzed, and the results pooled to reflect both within- and between-analysis variation, leading to valid statistical inferences that properly reflect the uncertainty due to missing values (11).
Outcomes of interest for multivariable Cox models were time to first biliary stricture, time to first grade 4 complication, and time from transplant to the earlier of chronic kidney disease (CKD) stage 4 or 5 including end-stage renal disease (ESRD). CKD was defined as having at least two eGFR measurements < 30 ml/min/1.73m2; to rule out acute kidney injury, we also required that the last eGFR measurement be < 30 ml/min/1.73m2. Date of onset of CKD was defined as the date of the first eGFR < 30 ml/min/1.73m2. Covariates tested in all models included recipient demographics, primary diagnosis, medical condition at transplant including MELD, and intraoperative characteristics such as cold ischemia time and length of operation. Transplant type (LDLT vs. DDLT) was included in all models regardless of significance; in some models DDLT was dichotomized based on whether or not the organ was donated after cardiac death (DCD, n=19), resulting in three transplant type categories (LDLT, non-DCD DDLT, and DCD-DDLT).
Models were selected using the method of best subsets on each of the five imputed datasets and the results were combined using the REGRESS macro in IVEware, which calculates the variances of parameter estimates, corrected for the between- and within-imputation variation. When different covariates were chosen in different imputation datasets, all chosen covariates across the five datasets were included in a combined model and backwards selection was used to remove covariates that did not meet the 0.05 significance level. All analyses were carried out using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Recipient demographics
Median follow-up time for the 1,036 recipients analyzed was 4.59 years (range 0–10.9). Follow-up time was longer for LDLT recipients compared to DDLT recipients (4.80 years vs. 4.19 years) but this difference was not statistically significant (log-rank p-value = 0.45). At the time of transplant, the average age of LDLT and DDLT recipients was 51.0 (SD=10.9) and 52.2 (SD = 10.4), respectively (Table 1). The majority of recipients were male and non-Hispanic with an average BMI between 26 and 27 kg/m2. HCV was the primary diagnosis in 45% and 40% of DDLT and LDLT, respectively. Recipients of LDLT and DDLT differed significantly by race (91% vs. 84% white, p=0.01), diagnosis of HCC (12% vs. 22%, p<0.0001), and cholestatic disease (25% vs. 20%, p=0.04), and disease severity at the time of transplant. DDLT recipients were more likely to have a higher physiologic MELD, more likely to be in the ICU, on a ventilator, or have ascites. Eighty-five percent of LDLT recipients had a physiologic MELD less than 21 at the time of the transplant compared to 57% of DDLT recipients. In addition, LDLT and DDLT recipients differed significantly on intraoperative characteristics such as cold ischemia time and duration of operation, with LDLT recipients having shorter cold ischemia times and longer operations (p<0.0001 in both cases).
Complication Probability by Transplant Type
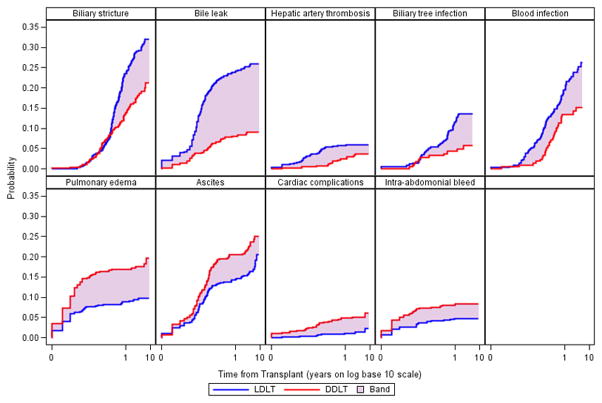
At least one complication occurred in 70% and 73% of DDLT and LDLT, respectively, highlighting the challenge of liver transplantation. Complications were further categorized as occurring with significantly higher probability in LDLT recipients, DDLT recipients, or occurring with similar probability in both groups based on the overall probability of first occurrence of the complication (Table 2). Five of the 36 tracked complications were more probable in recipients of LDLT, while four of the tracked complications were more probable in DDLT recipients. Complications more probable in LDLT recipients were predominantly biliary in nature. This included biliary leaks (LDLT probability 26% vs. DDLT probability 9%, p<0.0001), strictures (LDLT probability 32% vs. DDLT probability 21%, p=0.0002), and biliary tree infections (LDLT probability 14% vs. DDLT probability 6%, p=0.006). Hepatic artery thrombosis and blood infections also occurred with significantly higher probability in LDLT recipients. Localized intra-abdominal abscesses additionally occurred with significantly higher probability in LDLT in the first year post-transplant (6% compared to 3% in DDLT, p=0.02), but was not significantly different between the two groups, overall (p=0.06).
Table 2.
| In the first year | Overall | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| LDLT | DDLT | Log Rank p-value | LDLT | DDLT | Log Rank p-value | ||
| Significantly higher in LDLT over all follow-up | Bile leak/biloma | 0.24 | 0.08 | <.0001 | 0.26 | 0.09 | <.0001 |
| Blood infection¥ | 0.20 | 0.13 | 0.0519 | 0.26 | 0.15 | 0.0091 | |
| Biliary stricture | 0.23 | 0.14 | 0.0006 | 0.32 | 0.21 | 0.0002 | |
| Biliary tree infection | 0.11 | 0.04 | 0.0076 | 0.14 | 0.06 | 0.0062 | |
| Hepatic artery thrombosis | 0.06 | 0.02 | 0.0061 | 0.06 | 0.04 | 0.0378 | |
|
| |||||||
| Significantly higher in DDLT over all follow-up | Pulmonary edema | 0.09 | 0.17 | <.0001 | 0.10 | 0.36 | <.0001 |
| Ascites | 0.14 | 0.20 | 0.0132 | 0.21 | 0.25 | 0.0151 | |
| Cardiac complications | 0.01 | 0.05 | 0.0004 | 0.02 | 0.06 | 0.0008 | |
| Intra-abdominal bleeding | 0.05 | 0.08 | 0.0264 | 0.05 | 0.08 | 0.0190 | |
|
| |||||||
| Not significantly different in LDLT vs. DDLT over all follow-up | Respiratory arrest | 0.02 | 0.04 | 0.0770 | 0.02 | 0.11 | 0.0711 |
| Psychological complications | 0.09 | 0.12 | 0.2336 | 0.16 | 0.23 | 0.3866 | |
| Encephalopathy/hepatic coma | 0.05 | 0.05 | 0.8143 | 0.07 | 0.14 | 0.6642 | |
| Bowel obstruction | 0.03 | 0.02 | 0.4368 | 0.07 | 0.04 | 0.2952 | |
| C-difficile colitis | 0.02 | 0.02 | 0.3865 | 0.05 | 0.03 | 0.2491 | |
| Localized intra-abdominal abscess¥ | 0.06 | 0.03 | 0.0248 | 0.07 | 0.04 | 0.0641 | |
| GI bleeding | 0.05 | 0.03 | 0.0501 | 0.08 | 0.11 | 0.0594 | |
| GI tract infection | 0.06 | 0.08 | 0.2419 | 0.06 | 0.09 | 0.2840 | |
| Urinary tract infection | 0.10 | 0.14 | 0.1469 | 0.15 | 0.17 | 0.2293 | |
| Infection | 0.38 | 0.36 | 0.7305 | 0.43 | 0.45 | 0.8959 | |
| Wound infection | 0.06 | 0.05 | 0.5145 | 0.07 | 0.05 | 0.4973 | |
| Pulmonary infection | 0.08 | 0.07 | 0.6945 | 0.12 | 0.14 | 0.9338 | |
| Pleural effusion | 0.26 | 0.27 | 0.8401 | 0.30 | 0.32 | 0.7326 | |
| Re-exploration | 0.10 | 0.11 | 0.3953 | 0.10 | 0.12 | 0.4284 | |
| Liver infection/abscess | 0.05 | 0.03 | 0.3140 | 0.05 | 0.04 | 0.3811 | |
| Hernia | 0.08 | 0.07 | 0.8589 | 0.25 | 0.24 | 0.6200 | |
The following complications occurred with probability less than 5% in LDLT and DDLT recipients and were not significantly different between the two groups: Prolonged ileus, pneumothorax, neuropraxia, deep vein thrombosis, portal vein thrombosis, dehiscence, inferior vena cava thrombosis, CNS infection, pulmonary embolism, aspiration. Liver cirrhosis was significantly higher in DDLT, occurring with probability 5% in DDLT and 1% in LDLT (p=0.02 overall).
Within each grouping complications are ordered in descending order of absolute difference in probability between LDLT and DDLT.
Note blood infection was not significantly different between LDLT and DDLT in the first year post-transplant (p=0.05) but is classified as significantly higher in LDLT based on overall probability of occurrence, while localized intra-abdominal abscess was significantly higher in LDLT in the first year but is classified as not significantly different between LDLT and DDLT for similar reasons.
Whereas technical complications were more probable in LDLT, complications potentially related to graft issues or ischemia reperfusion were more probable in DDLT recipients. We found in DDLT recipients ascites, pulmonary edema, intra-abdominal bleeding, and cardiac complications were more probable, with the largest difference occurring in pulmonary edema, occurring with a probability of 36% in DDLT compared to 10% in LDLT (p<0.0001). All of the differences mentioned above were present in the first year post-transplant except for blood infection, which occurred with a probability of 20% in LDLT compared to 13% in DDLT in the first year post-transplant (p=0.05).
Figure 1 shows the unadjusted Kaplan-Meier estimate of the probability of the first occurrence of the nine complications that occurred with significantly different probabilities in LDLT compared to DDLT. Time in years from transplant is shown on the log base 10 scale to highlight differences in the first year post-transplant. In the case of biliary stricture, biliary tree infection, and blood infection, differences in probability between LDLT and DDLT do not manifest until later into the first year post-transplant, while differences in other complications can be seem almost immediately post-transplant. In addition, biliary stricture, blood infection, and ascites continue to occur for the first time with relatively high probability well after the first year post-transplant.
Figure 1.
Probability of Complications Significantly Different between LDLT and DDLT over all Follow-up
In 27 of the 36 complications tracked in the study there were no significant differences in the overall probability of occurrence between the types of transplants. Infections were the most common complication that recipients of liver transplant faced, occurring with a probability of 43% in LDLT and 45% in DDLT (p=0.90, Table 2). Pleural effusion, hernia, and psychological complications also occurred with a probability of at least 20% in either LDLT or DDLT. While some complications occurred for the first time with high probability in the first year but relatively low probability after that, some complications continued to occur with high probability well after the first year post-donation. This was most notable in the case of hernias, which occurred with a probability of 7%–8% in the first year but 24%–25% overall, indicating high probability of first occurrence after the first year. Infection, ascites, biliary strictures, and psychological complications also continued to occur for the first time with relatively high probability after the first year post-transplant.
Biliary Complications
The probability of biliary leak was three times greater in the first year post-LDLT compared to DDLT. Nearly all biliary leaks occurred in the first year and there were no differences in leaks after the first year (Supplementary Table 1). There was a 39% reduction in the probability of biliary stricture with DDLT versus LDLT in the first year (Table 2). Biliary strictures continued to occur in LDLT and DDLT after the first year with relatively high probability (Figure 1) although there was no significant difference between DDLT and LDLT after the first year. In fact, there was a 50% increase in the unadjusted probability of biliary strictures in DDLTs after the first year. After adjusting for other significant factors related to the first occurrence of biliary stricture including duration of operation, and dividing the DDLT group into those who received a DCD organ (n=19) and those who did not, we found that while the risk of biliary strictures in non-DCD DDLT recipients was 43% lower than in LDLTs (95% CI = 0.41–0.77, p=0.0004, Table 3), DCD DDLT had a much higher risk of biliary strictures compared to LDLT recipients (HR = 2.15, 95% CI = 1.07–4.34, p=0.03). Additionally, we found that liver transplant recipients at centers with the higher volumes of LDLT in a given year appeared to have lower biliary stricture risk (HR = 0.82, 95% CI = 0.97–0.99, 0.04).
Table 3.
Time from Transplant Cox Models Censored at earliest of graft failure or last follow-up
| Hazard Ratio | 95% Confidence Interval for the Hazard Ratio | P-value | ||
|---|---|---|---|---|
| Biliary Stricture (n=223 events) | Transplant Type (ref = LDLT) | |||
| DDLT (non-DCD) | 0.57 | (0.41, 0.77) | 0.0004 | |
| DDLT (DCD) | 2.15 | (1.07, 4.34) | 0.0324 | |
| Duration of operation (per 100 minutes) | 1.13 | (1.02, 1.24) | 0.0140 | |
| Center Living Donor Volume by Year (Per 10 cases) | 0.82 | (0.67, 0.99) | 0.0400 | |
|
| ||||
| CKD or ESRD (n=39 events)* | Transplant Type: DDLT vs. LDLT | 2.45 | (1.89–5.05) | 0.0152 |
| Serum Creatinine at Transplant | 1.50 | (1.17, 1.93) | 0.0015 | |
| Ascites at Transplant | 2.34 | (1.09, 5.03) | 0.0290 | |
| History of TIPSS | 2.60 | (1.13, 5.96) | 0.0241 | |
|
| ||||
| Grade 4 Complication (n=101 events) | Transplant Type (ref = LDLT) | |||
| DDLT (non-DCD) | 0.89 | (0.58, 1.38) | 0.6024 | |
| DDLT (DCD) | 2.65 | (0.93, 7.52) | 0.0660 | |
| Age at Transplant (per 10 years) | 1.20 | (0.99, 1.46) | 0.0626 | |
| On Dialysis at Transplant | 4.04 | (1.89, 8.64) | 0.0003 | |
| Ascites at Transplant | 1.56 | (1.03, 2.36) | 0.0357 | |
| Duration of operation (per 100 minutes) | 1.26 | (1.11, 1.44) | 0.0003 | |
Additionally censored at last eGFR measurement. Excludes patients on dialysis at transplant unless showed evidence of post-transplant recovery.
Differences in renal function after transplantation
While the frequency of CKD or ESRD as defined above was relatively low in our population, occurring in only 11 LDLT recipients and 28 DDLT recipients, we observed that the risk of developing CKD or ESRD post-liver transplant was 2.5 times higher for DDLT recipients compared to LDLT recipients (95% CI = 1.89–5.05, p=0.02). This effect was adjusted for the level of serum creatinine at transplant, implying that among LDLT and DDLT recipients with the same level of serum creatinine at transplant, LDLT recipients have a lower risk of developing CKD or ESRD post-transplant. This holds for both recipients with relatively high levels of serum creatinine, indicating renal dysfunction at the time of transplant, as well as for recipients with normal renal function at transplant as measured by serum creatinine. The model was also adjusted for presence of ascites at transplant, and history of TIPSS at transplant, which were also significantly associated with the risk of developing CKD or ESRD post-liver transplant (Table 3).
Risk of Grade 4 Complications
Using the Clavien grading system, grade 4 complications were defined as those that resulted in retransplant or death. Comparing DDLT to LDLT, when analyzing cases after the twentieth LDLT case at each center, there was no difference in risk of grade 4 complications between LDLT and non-DCD DDLT recipients (HR = 0.89, 95% CI = 0.58–1.38, p=0.60). Other factors significantly associated with greater risk of grade 4 complications were older age at the time of transplant, disease severity indicators such as being on dialysis and having ascites at the time of transplant, and longer duration of the operation.
Probability of Resolution of Complications by Transplant Type
We sought to determine if complications had different probabilities of resolution depending on transplant type (Figure 2a and 2b). We did not find significant differences in unadjusted probability of resolution between the types of transplant, from the time of complication onset. Encephalopathy and infections had a high probability of resolution in the first year post-onset. Only half of post-transplant ascites resolved within 30 days of transplant and in one-third of patients who had at least one occurrence of post-transplant ascites, it lasted for more than 90 days. Between 72%–73% of biliary strictures resolved within one year, and the remainder continued to reach resolution years after occurrence (Figure 2b). Patients with psychological complications or hernias had the lowest probabilities of resolution. Approximately 66%–67% of hernias resolved within a year, and while the probability of resolution increased slightly after 1 year, the overall probability of resolution was around 74%–75%. Psychological complications had a lower probability of resolution in LDLT compared to DDLT (56% compared to 100%), but this result did not reach statistical significance (p=0.06).
Figure 2.
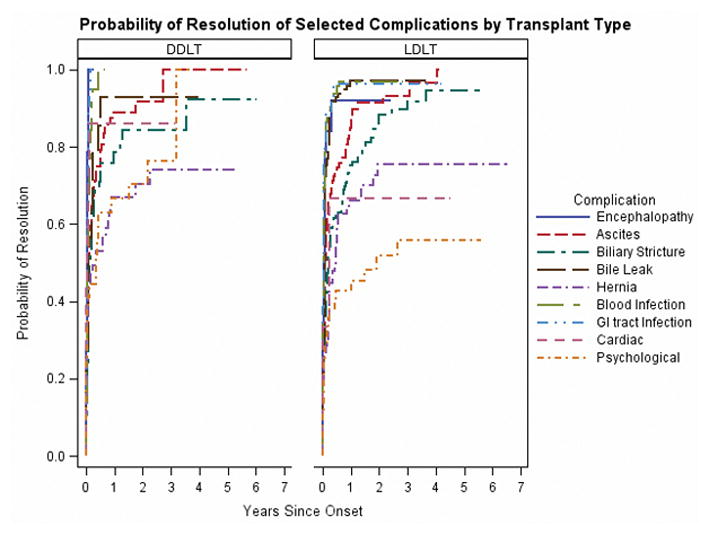
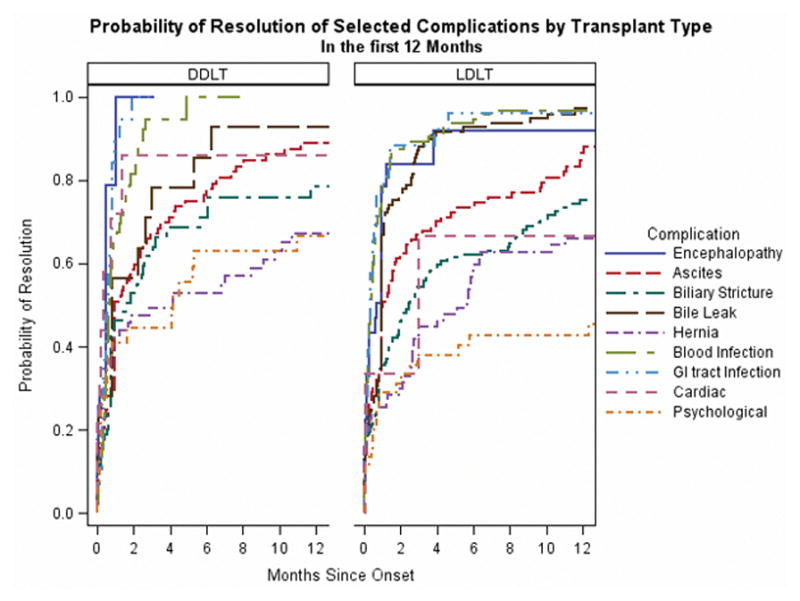
Figure 2a: Probability of Resolution of Selected Complications by Transplant Type
Figure 2b: Probability of Resolution of Selected Complications by Transplant Type in the first 12 Months
Discussion
LDLT has been previously shown to be associated with improved overall mortality among wait-listed candidates (2). Additionally, we have shown that post-transplant patient and graft survival after LDLT and DDLT are equivalent in recipients from the A2ALL consortium (12). Despite these published findings, LDLT volume continues to be very modest in the United States. Aside from the potential for donor morbidity, a likely contributor is the real and perceived higher technical difficulty of the recipient procedure. However, among experienced LDLT centers in the A2ALL consortium, we found no significant overall difference between DDLT and LDLT recipients in the probabilities of the majority of 36 post-operative complications that we tracked in this study.
We found important differences in the probability and risk factors for a minority of complications, with some being more likely in LDLT recipients and others in DDLT recipients. LDLT recipients had a significantly higher probability of technical complications including bile leaks, biliary strictures, and biliary tree infections, although the risk of biliary stricture was much lower in LDLT recipients when compared to the subset of DDLT recipients who received DCD organs. Some complications were significantly more probable in DDLT recipients: ascites, pulmonary edema, cardiac complications, and intra-abdominal bleeding. We also found an important and significantly higher risk for CKD (including ESRD) among DDLT recipients regardless of the level of renal function at transplant. The majority of the differences in complication probabilities were seen in the first year post-transplant and not in later post-transplant years, as expected. However, some complications such as biliary strictures, hernias, and psychological complications occurred for the first time many years after transplant. The probability of resolution of complications was not found to be different between the two types of transplant.
Some complications occurring with higher probability in DDLT recipients may be related to an enhanced systemic inflammatory response to ischemia-reperfusion injury in the deceased donor setting. This could contribute to renal dysfunction, and to cardiac and pulmonary issues that were observed more commonly after DDLT. On the other hand, ascites, pulmonary edema, cardiac complications, and recurrent cirrhosis were also observed more frequently after deceased donor liver transplant and may be related to higher severity of illness among the DDLT recipients.
We found that center experience beyond the first 20 LDLT cases was not associated with further improvement in the probability of biliary complication occurrence. This is similar to some single center studies(13). We did find a trend to decrease the probability of biliary stricture in centers performing the highest number of living donor transplants per year, suggesting that greater currency of experience, rather than cumulative experience, may play a role in lowering technical complication rates in LDLT recipients.
Small for size syndrome (SFSS) was initially described in LDLT with cholestasis, renal failure, and ascites after left lateral segment (LLS) and left lobe grafts in older children(14). In our study, the majority of recipients received right lobe grafts, where the volume of hepatic parenchyma was generally thought to be adequate to support recipient metabolic and functional demands. Although we did not track SFSS as a syndromic complication, the components of renal failure and ascites were observed to be less rather than more likely in LDLT recipients.
The majority of complications resolved in recipients of both types of liver transplants. Once a complication occurred, the time to and overall likelihood of resolution were similar in LDLT and DDLT recipients. However, the time to resolution was quite lengthy for certain complications. Some complications, such as hernia, psychological decompensation, and ascites, did not achieve resolution. Although patients with treated depression have been shown to have equivalent medical outcomes to non-depressed patients, there may be an impact on long-term post-transplant quality of life (15).
Our finding of a significantly lower risk of severe renal dysfunction (CKD or ESRD) in the months and years after LDLT is novel and potentially important, because of its well-established deleterious effect on mortality following liver transplant (16). Chronic renal dysfunction prior to transplant may render the kidney more prone to post-transplant injury (17). Acute kidney injury has also been shown to be a risk factor for chronic kidney disease (18–20). Recipients of DDLT with higher calculated MELD may have had a higher incidence of hepatorenal syndrome (HRS). We were not able to determine the incidence of hepatorenal syndrome (HRS) prior to transplant, and therefore were not able to test the effect of HRS on the subsequent development of post-transplant CKD. However, recent single center studies have found equivalent renal outcomes in LDLT and DDLT patients with HRS (21, 22). Our analysis adjusted for serum creatinine at the time of transplant, and, although serum creatinine is an imperfect proxy for the magnitude of pre-existing renal dysfunction in patients with liver disease, there is no reason to think that serum creatinine differentially represents renal dysfunction in LDLT versus DDLT candidates, However, before drawing strong inference from our findings, they should be validated by other groups.
In summary, liver transplantation continues to be associated with a high complication rate regardless of donor source. Adding to the known improvement in overall survival associated with use of living donor grafts, this study has demonstrated an important association of LDLT with lower risk of post-transplant renal deterioration. Unfortunately, biliary complications remain a persistent and vexing issue after LDLT and the incomplete resolution of these and other complications, in both LDLT and DDLT recipients, remain important targets for improvement.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531, and U01-DK62536). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study
- LDLT
Living donor liver transplant
- DDLT
Deceased donor liver transplant
- MELD
Model for End-Stage Liver Disease
- HCC
Hepatocellular carcinoma
- DCD
Donation after cardiac death
- HR
Hazard ratio
- CI
Confidence interval
Footnotes
- Columbia University Medical Center, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-Is: Robert S. Brown, Jr., MD, MPH, James Guarrera, MD, FACS, Martin R. Prince, MD, PhD, Benjamin Samstein, MD, Elizabeth Verna, MD, MS; Study Coordinators: Taruna Chawla, MD, Scott Heese, MPH, Theresa Lukose, PharmD, Rudina Odeh-Ramadan, PharmD, Jonah Zaretsky, BS.
- Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-Is: Talia Baker, MD, Laura M. Kulik, MD, Daniela P. Ladner, MD; Study Coordinator: Patrice Al-Saden, RN, CCRC.
- University of California Los Angeles, Los Angeles, CA (DK62496): PI: Johnny C. Hong, MD; Co-I: Ronald W. Busuttil, MD, PhD; Study Coordinator: Janet Mooney, RN, BSN. University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-I: Norah A. Terrault, MD, MPH; Study Coordinator: Dulce MacLeod, RN.
- University of Colorado Denver, Aurora, CO (DK62536): PI: James R. Burton, Jr., MD; CoIs: Gregory T. Everson, MD, FACP, Igal Kam, MD, James Trotter, MD; Study Coordinators: Carlos Garcia, RN, BS, Anastasia Krajec, RN. University of Michigan Health System, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD, FACS; DCC Staff: Mary Akagi, MS, CCRP, Douglas R. Armstrong, BSN, MS, Abby Brithinee, BA, Margaret Hill-Callahan, BS, LSW, Lisa Holloway, BS, CCRC, Terese A. Howell, BS, CCRC, Brenda W. Gillespie, PhD, Beth Golden, BScN, Anna S.F. Lok, MD, Monique Lowe, MSI, Akinlolu O. Ojo, MD, PhD, Samia Shaw, AAIT, Abigail Smith, MS, Robert A. Wolfe, PhD.
- University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD, MPH; Study Coordinator: Tracy Russell, MA.
- University of Pennsylvania, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD; Co-Is: Kim M. Olthoff, MD, FACS, K. Rajender Reddy, MD, Mark A. Rosen, MD, PhD; Study Coordinators: Brian Conboy, PA, MBA, Mary Kaminski, PA-C, Debra McCorriston, RN, Mary Shaw, RN, BBA.
- University of Virginia (DK62484): PI: Carl L. Berg, MD; Co-I: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN.
- Virginia Commonwealth University - Medical College of Virginia, Richmond, VA (DK62531): PI: Robert A. Fisher, MD, FACS; Co-Is: Adrian Cotterell, MD, FACS, R. Todd Stravitz, MD, FACP; Study Coordinators: April Ashworth, RN, BSN, Joanne Davis, RN, Ede Fenick, RN, Andrea Lassiter, BS, Cheryl Rodgers, RN, Jose Rodriguez, MPH, Luke Wolfe, MS.
- National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Edward Doo, MD, James E. Everhart, MD, MPH, Jay H. Hoofnagle, MD, Stephen James, MD, Patricia R. Robuck, PhD, Leonard B. Seeff, MD, Rebecca J. Torrance, RN, MS.
The supplemental data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
Supplementary Table 1: Probability of Specific Complications in Recipients of LDLT and DDLT
References
- 1.Shah SA, Levy GA, Greig PD, Smith R, McGilvray ID, Lilly LB, et al. Reduced mortality with right-lobe living donor compared to deceased-donor liver transplantation when analyzed from the time of listing. Am J Transplant. 2007;7(4):998–1002. doi: 10.1111/j.1600-6143.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 2.Berg CL, Merion RM, Shearon TH, Olthoff KM, Brown RS, Jr, Baker TB, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011;54(4):1313–21. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8(12):2569–79. doi: 10.1111/j.1600-6143.2008.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaprak O, Dayangac M, Akyildiz M, Demirbas T, Guler N, Bulutcu F, et al. Biliary complications after right lobe living donor liver transplantation: a single-centre experience. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2012;14(1):49–53. doi: 10.1111/j.1477-2574.2011.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichman TW, Katchman H, Tanaka T, Greig PD, McGilvray ID, Cattral MS, et al. Living donor versus deceased donor liver transplantation: a surgeon-matched comparison of recipient morbidity and outcomes. Transpl Int. 2013;26(8):780–7. doi: 10.1111/tri.12127. [DOI] [PubMed] [Google Scholar]
- 6.Merion RM, Shearon TH, Berg CL, Everhart JE, Abecassis MM, Shaked A, et al. Hospitalization rates before and after adult-to-adult living donor or deceased donor liver transplantation. Ann Surg. 2010;251(3):542–9. doi: 10.1097/SLA.0b013e3181ccb370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoehn RS, Wilson GC, Wima K, Hohmann SF, Midura EF, Woodle ES, et al. Comparing Living Donor and Deceased Donor Liver Transplant: A Matched National Analysis from 2007–2012. Liver Transpl. 2014 doi: 10.1002/lt.23956. [DOI] [PubMed] [Google Scholar]
- 8.Clavien PA, Camargo CA, Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220(2):109–20. doi: 10.1097/00000658-199408000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavien PA, Sanabria JR, Mentha G, Borst F, Buhler L, Roche B, et al. Recent results of elective open cholecystectomy in a North American and a European center. Comparison of complications and risk factors. Ann Surg. 1992;216(6):618–26. doi: 10.1097/00000658-199212000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111(5):518–26. [PubMed] [Google Scholar]
- 11.Raghunathan TELJ, Van Hoewyk J, Solenberg P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27(1):85–95. [Google Scholar]
- 12.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Annals of Surgery. 242(3):314–23. doi: 10.1097/01.sla.0000179646.37145.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomposelli JJ, Verbesey J, Simpson MA, Lewis WD, Gordon FD, Khettry U, et al. Improved survival after live donor adult liver transplantation (LDALT) using right lobe grafts: program experience and lessons learned. Am J Transplant. 2006;6(3):589–98. doi: 10.1111/j.1600-6143.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- 14.Emond JC, Renz JF, Ferrell LD, Rosenthal P, Lim RC, Roberts JP, et al. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Annals of Surgery. 1996;224(4):544–52. doi: 10.1097/00000658-199610000-00012. discussion 52–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogal SS, Dew MA, Fontes P, DiMartini AF. Early treatment of depressive symptoms and long-term survival after liver transplantation. Am J Transplant. 2013;13(4):928–35. doi: 10.1111/ajt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. The New England journal of medicine. 2003;349(10):931–40. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 17.Chok KS, Fung JY, Chan SC, Cheung TT, Sharr WW, Chan AC, et al. Outcomes of living donor liver transplantation for patients with preoperative type 1 hepatorenal syndrome and acute hepatic decompensation. Liver Transpl. 2012;18(7):779–85. doi: 10.1002/lt.23401. [DOI] [PubMed] [Google Scholar]
- 18.Leithead JA, Armstrong MJ, Corbett C, Andrew M, Kothari C, Gunson BK, et al. Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation. Transpl Int. 2013;26(11):1116–25. doi: 10.1111/tri.12175. [DOI] [PubMed] [Google Scholar]
- 19.Velidedeoglu E, Bloom RD, Crawford MD, Desai NM, Campos L, Abt PL, et al. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation. 2004;77(4):553–6. doi: 10.1097/01.tp.0000114609.99558.41. [DOI] [PubMed] [Google Scholar]
- 20.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–85. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 21.Goldaracena N, Marquez M, Selzner N, Spetzler VN, Cattral MS, Greig PD, et al. Living vs. Deceased Donor Liver Transplantation Provides Comparable Recovery of Renal Function in Patients With Hepatorenal Syndrome: A Matched Case-Control Study. Am J Transplant. 2014;14(12):2788–95. doi: 10.1111/ajt.12975. [DOI] [PubMed] [Google Scholar]
- 22.Lee JP, Kwon HY, Park JI, Yi NJ, Suh KS, Lee HW, et al. Clinical outcomes of patients with hepatorenal syndrome after living donor liver transplantation. Liver Transpl. 2012;18(10):1237–44. doi: 10.1002/lt.23493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.