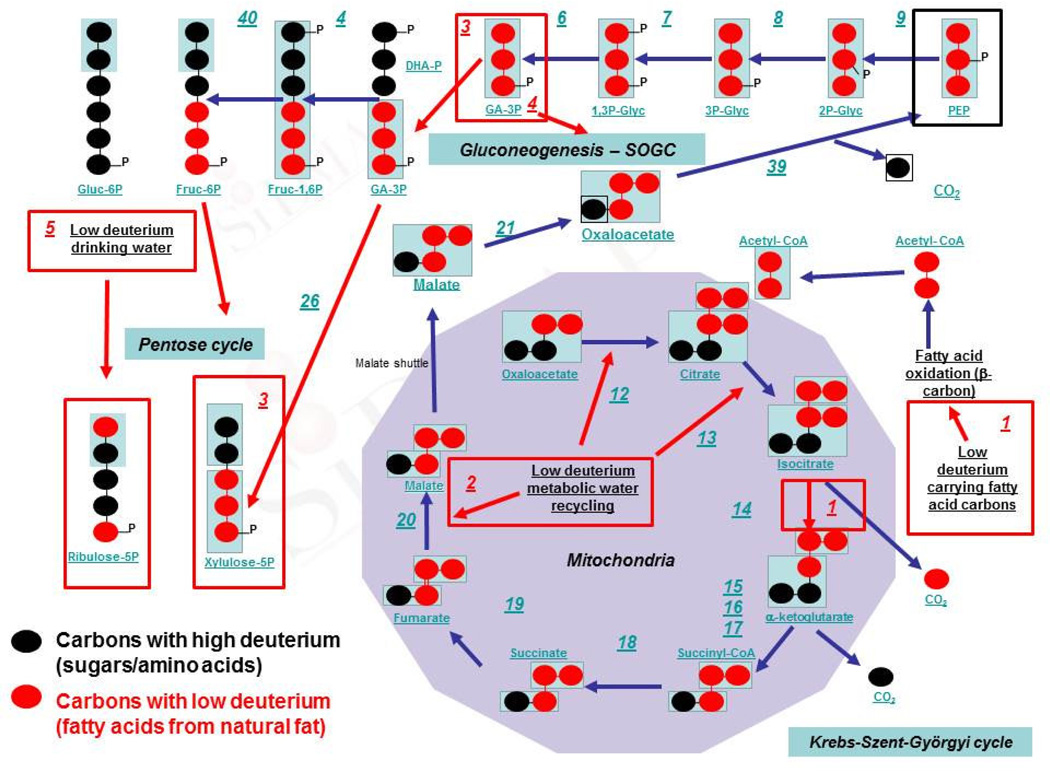
Figure 1.
Comparison of metabolic profile changes associated with 1) natural deuterium depletion by low deuterium fatty acid oxidation. Avastin® and Glivec® exert similar effect and require intact mitochondria for efficacy [49–51] (Red boxes No1), and 2) low deuterium metabolic water recycling from the mitochondrial matrix during citrate, isocitrate and malate formation; the target of fumarate hydratase activation [36,37] and hyperbaric oxygen treatment combined with a ketogenic diet [52] (Red box No2). Mitochondrial shuttles, such as the malate shuttle, pass low deuterium carrying fatty acid carbons to gluconeogenesis, where glyceraldehyde-3-phosphate becomes the source of extensive carbon exchange reactions [53] for the non-oxidative pentose cycle to maintain low deuterium saturation in C3’-C5’ pentose sugar carbon positions in RNA and DNA (Red box No3). These are the carbon sites where DNA stability, radiation- and chemotherapy derived hydroxyl radical sensitivities are regulated by hydrogen/deuterium [23,24] due to primary and secondary intrinsic isotope effects; as well as partially by collective proton tunneling [15,16]. Besides the C3’–C5’ nucleic acid sugar backbone fragment, de novo nucleic acid base syntheses, hydrogen bonding and deuterium channeling into hydrogen bonds are controlled by the serine oxidation glycine cleavage single carbon cycle pathways [38; SOGC] (Red box No4). When tumor cells revert to the Warburg phenotype and reductive carboxylation-driven mitochondria, deuterium depletion in free (drinking) water becomes the only deuterium depleting mechanism for specific carbon sites in nucleic acid backbone sugars and the bases (Red box No5). (Blue arabic numbers are enzyme identifiers also found in [54])