Table 1.
Optimization of the Pictet–Spengler cyclization stepa
 | |||||
|---|---|---|---|---|---|
| Entrya | Acid | Solvent | Tempb (°C) | Time (h) | Convc (%) |
| 1 | HCOOH | MeOH | rt | 18 | — |
| 2 | HCOOH | MeOH | 80 °C | 18 | — |
| 3 | HCOOH | — | rt | 18 | — |
| 4 | HCOOH | — | 80 °C | 18 | — |
| 5 | CF3COOH | MeOH | 80 °C | 18 | Traces |
| 6 | CF3COOH | CH2Cl2 | rt | 18 | Traces |
| 7 | CF3COOH | CH3CN | 60 °C | 18 | Traces |
| 8 | CF3COOH | Toluene | 80 °C | 18 | Traces |
| 9 | CF3COOH | — | 50 °C | 18 | Traces |
| 10 | CH3SO3H | Toluene | 80 °C | 18 | Traces |
| 11 | CH3SO3H | MeOH | 80 °C | 18 | Traces |
| 12 | CH3SO3H | — | 80 °C | 18 | Traces |
| 13 | CH3SO3H | — | rt | 18 | 67d |
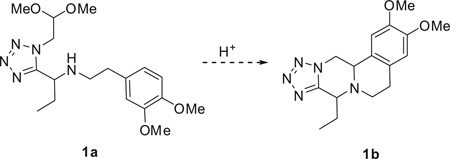
Reaction conditions: 1a (0.3 mmol), acid (30.0 mmol), solvent (3.0 mL).
The reaction was performed under classical heating conditions.
Determined by SFC–MS analysis of crude reaction mixture.
Isolated product.
