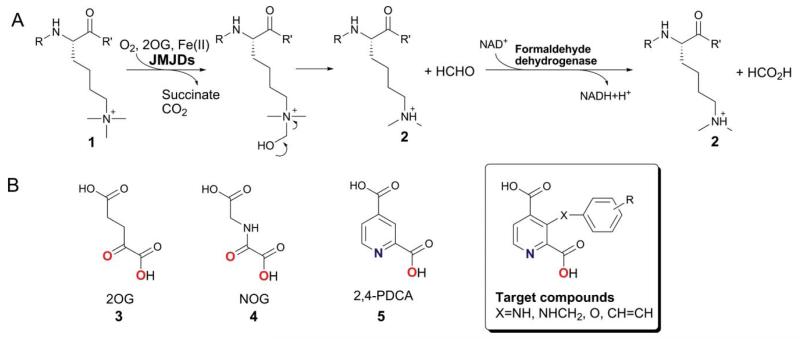
Scheme 1.
A. The Nε-methyl lysine demethylation reaction catalysed by the 2OG-dependent JMJD2 histone demethylases, which preferentially act on tri- and di-Nε-methylated lysines. Demethylation of Nε-trimethyllysyl residues (1) proceeds via hydroxylation to give an unstable hemiaminal intermediate, which collapses spontaneously to release the demethylation product 2. The formation of the by-product formaldehyde can be measured spectrophotometrically using formaldehyde dehydrogenase and NAD+ in a coupled enzymatic assay. B. Structures of the 2OG cosubstrate (3), its analogues NOG (4) and 2,4-PDCA (5), and the targeted C-3 substituted 2,4-PDCA derivatives. The ligand atoms involved in chelation of the ferrous iron are shown in red and blue.