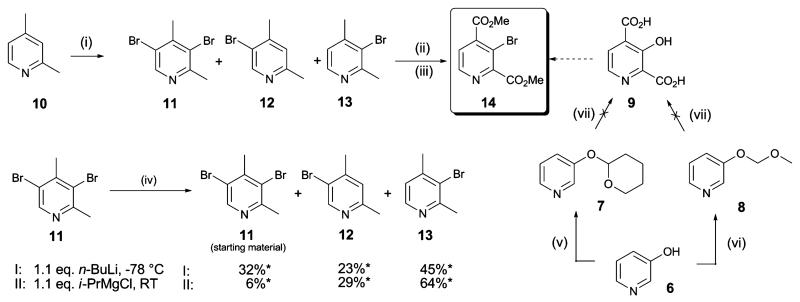
Scheme 2.
Synthesis of 3-bromopyridine 14. Reagents and conditions: (i) Br2 (0.9 eq.), 20% oleum, 165 °C, 24 h, 11 (12%), 12 (22%), 13 (35%); (ii) KMnO4 (5 eq.), NaOH (0.7 eq.), H2O, 110 °C, 3 h; (iii) cat. H2SO4, MeOH, reflux, 24 h, 53%; (iv) n-BuLi (1.1 eq.), THF, −78 °C, 1 h, or i-PrMgCl (1.1 eq.), THF, RT; then MeOH workup; (v) tetrahydropyran-2-ol (1.1 eq.), PPh3 (1.1 eq.), diisopropyl azodicarboxylate (1.1 eq.), RT, 2 h, 46%;23 (vi) methoxymethyl chloride (1.05 eq.), KOtBu (1.1 eq.), DMF–CH3CN, RT, 1 h;26 (vii) base (1.5 eq.), then excess CO2(s); base (1.5 eq.), then excess CO2(s); then HCl/dioxane; *Ratios as determined by 1H NMR.