Abstract
The present meta-analysis aimed to evaluate the current evidence for the use of α1-blockers in relieving ureteric stent-related symptoms (USS). Electronic databases, including PubMed, Embase and Cochrane Library, were searched and two independent reviewers identified relevant parallel randomized controlled trials (RCTs), assessed trial quality and extracted data. Review Manager (version 5.2) was used to conduct a meta-analysis of the data. Significant advantages were demonstrated in the treatment group based on International Prostate Symptom Score (IPSS), voiding symptom sub-scores [mean difference (MD), −2.66; 95% confidence interval (CI), (−4.36, −0.96)], Ureteral Stent Symptom Questionnaire (USSQ) urinary symptoms score (MD, −5.84; 95%CI, −9.35 to −2.33), IPSS quality of life score (MD, −1.46; 95%CI, −2.64 to −0.28) USSQ quality of life score (MD, −0.69; 95%CI, −1.10 to −0.28), USSQ pain score (MD, −3.97; 95%CI, −5.52 to −2.42), Visual Analog Pain Scale (MD, −1.53; 95%CI, −2.25 to −0.80) and USSQ general health score (MD, −1.82; 95%CI, −2.47 to −1.18). No significant differences were detected from the following results: IPSS storage symptom sub-score (MD, −0.93; 95%CI, −2.28 to 0.43), USSQ sexual matters score (MD, −0.10; 95%CI, −0.79 to 0.59), USSQ work performance score (MD, 1.64; 95%CI, −2.18 to 5.47) and USSQ additional problems score (MD, −2.02; 95%CI, −4.55 to 0.52). However, significant between-trial heterogeneity was detected following statistical analysis and there were insufficient data to trace its source. The existing RCT data supported the hypothesis that α1-blockers beneficially influence pain, urinary symptoms and the quality of life of patients with an indwelling ureteral stent.
Keywords: α1-blockers, ureteral stent, quality of life
Introduction
The insertion of a ureteral stent is a common therapeutic procedure in the field of urology, with methods including ureteroscopy, ureteroscopy lithotripsy (URS), extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy and ureterolithotomy (1,2). The internal drainage provided by ureteral stents may prevent ureteral obstruction caused by stone fragments or edema, and promote the repair of severe mucosal injury and ureteral perforations (3,4). However, complications of indwelling ureteral stents are common in patients, including flank or suprapubic pain, urinal frequency and urgency, dysuria, hematuria, urinary tract infection, fever, and other voiding symptoms. These symptoms impact patients' health and quality of life to varying degrees (5); therefore, whether it is necessary to routinely insert a stent following a ureteroscopy for urinary calculi remains controversial (6–8). A systematic review by Makarov et al (8) indicated that routine ureteral stenting following an uncomplicated ureteroscopy reduced the risk of operation-related complications in patients. Furthermore, Damiano et al (7) concluded that routine stent placement was advised following uncomplicated ureteroscopic lithotripsy without ureteral dilation, particularly when the stones were incompletely fragmented.
Various ureteral stents and therapeutic agents have been investigated for their potential to reduce ureteric stent-related symptoms (USS), including ureteral stents composed of softer biomaterials, stents with various sizes and shapes, and drug eluting ureteral stents with various coatings (9–11). Furthermore, nonsteroidal anti-inflammatory drugs, calcium channel antagonists, α-adrenoceptor blockers and anticholinergics have been investigated in various clinical trials to alleviate USS in patients following ureteral stenting (9,12,13). Since a perfect ureteral stent that does not cause discomfort remains elusive, a fast and efficient approach to relieve stent-related symptoms is required (9). Joshi et al (5) developed the Ureteral Stent Symptom Questionnaire (USSQ) in order to evaluate the symptoms and impact on the quality of life of patients following ureteral stents, which included 38 scoring items over six sections, covering: Urinary symptoms, pain, general health, work performance, and sexual matters with additional problems. Additional methods, including the Visual Analog Pain Scale (VAPS), International Prostate Symptom Score (IPSS) and the Short-form 36 Health Survey Scale (SF-36) have been applied to evaluate USS in relevant studies (14,15). Previous studies have demonstrated that α1-blockers have a significant impact on patients with USS (13,16). α1-blockers inhibit basal tone and decrease peristaltic frequency and amplitude, thus improving stone-free rate and shortening the time to stone expulsion in patients with ureteral stones. Therefore, α1-blockers may alleviate USS by relaxing ureteral smooth muscle (17).
The present systematic review and meta-analysis was conducted in order to broadly evaluate the available evidence for the use of α1-blockers in relieving USS.
Materials and methods
Search strategy
PubMed (1966–2014), Embase (1974–2014), the Cochrane Library (issue 3; 2014), Chinese Biomedicine Literature Database (1978–2014; http://www.sinomed.ac.cn/zh/), Chinese Technological Periodical Full-text Database (1989–2014; http://oldweb.cqvip.com/ZK/), and Chinese Periodical Full-text Database (1994–2014; http://www.cnkie.net/) were searched for randomized controlled trials (RCTs) that investigated α1-adrenoceptor antagonists for the prevention and treatment of USS using the following key words: Ureteral stent, double-J stent, α1-adrenoceptor antagonists, α1-blockers, terazosin, tamsulosin, doxazosin, and alfuzosin. In order to search key Chinese journals within related fields, the Google search engine was used to manually locate studies and references. Furthermore, the reference lists of the included studies and reviews were manually searched by experts in the field. Unpublished studies were not included and no limits based on language were imposed.
Study selection
The described search strategy was initially used to obtain titles and abstracts of RCTs that met the inclusion criteria. Subsequently, the titles and abstracts were screened by two independent reviewers (Long Cheng and Gang Weng), who discarded inapplicable studies, and assessed the retrieved titles and abstracts of all the relevant RCTs in order to confirm whether they met the inclusion criteria. Disagreements were resolved in consultation with a third reviewer.
Data extraction
Data was independently extracted by the two reviewers using standard data extraction forms. For continuous outcomes, the number of participants, mean outcome value and standard deviation (SD) was required for each group. In the event that SD was not declared, the authors were contacted directly. If the authors failed to respond, the SD was calculated using the P-value or imputed from other RCTs in the
Inclusion criteria
Parallel RCTs that compared α1-blocker administration with a concurrent placebo or blank control as a treatment for USS were eligible for inclusion in the present review. Indications and locations of ureteral stents, the follow-up periods and the outcome measures were unrestricted. Case control studies or other non-RCTs were excluded.
Types of outcome measures
Any tool used to evaluate the primary outcome measures, including urinary symptoms, pain and quality of life was deemed acceptable. In the included studies, USSQ, IPSS, VAPS, SF-36 and Overactive Bladder Questionnaire (OABq) were applied to assess USS. VAPS was graded from 1 (minimal or no symptoms) to 10 (symptoms of maximal severity). SF-36 and OABq were also observed and analyzed, although they were only used in two studies. When the outcomes were reported at multiple time points, the latest time point prior to the removal of the ureteral stent was used.
Methodological quality assessment
The Cochrane Review Manager Risk of Bias tool (version 5.2; The Cochrane Collaboration, Oxford, UK) was used to evaluate the methodological quality of the included RCTs. Seven parameters were included: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each parameter was answered with one of the following: ‘Low’, low risk of bias; ‘unclear’, either lack of information or uncertainty over the potential for bias; and ‘high’, high risk of bias.
Data synthesis and analysis
Data were synthesized and analyzed using Review Manager (version 5.2; The Cochrane Collaboration). For all outcomes, mean difference (MD) was used to summarize the effects of α1-blockers. Heterogeneity was assessed by examining the clinical characteristics of the included studies and subsequent formal statistical testing with χ2 and I2. For continuous outcomes, a fixed-effect analytical model was used to calculate the MD and the 95﹪ CI. If significant heterogeneity was detected among the studies, according to I2 values >50%, a random effects statistical model was used. Between-trial heterogeneity was determined by I2 values >50%, and potential sources of heterogeneity were investigated. Subgroup analyses based on: Study quality, population, treatment duration, outcome assessment timing or additional analgesics, and antibiotics use, were used to investigate potential sources of heterogeneity. In addition, sensitive analyses were performed in order to test the robustness of the results.
Results
Search results
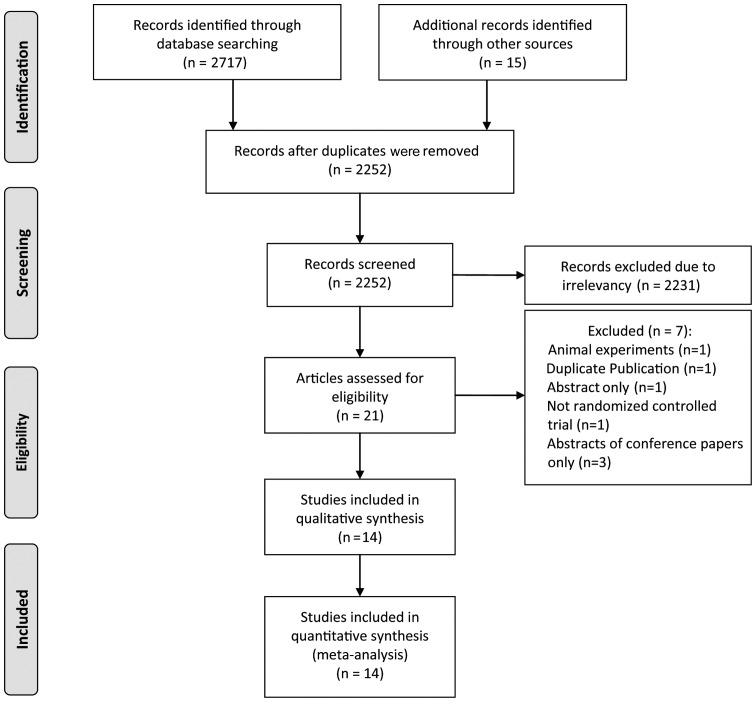
The selection process was depicted in Fig. 1. A total of 21 potentially eligible studies were identified and seven studies were subsequently excluded for not fulfilling the inclusion requirements, one of which was not a RCT (20). A Russian article published in Urologiia met the inclusion criteria according to the English abstract in PubMed; however it was subsequently excluded, since the full text could not be located (21). A total of 14 RCTs including 1,075 patients were included in the present meta-analysis (13,15,16,22–32).
Figure 1.
Flowchart for identifying and selecting studies for the systematic review.
Characteristics of the included studies
Table I outlines the characteristics of each study included in the present meta-analysis. All included studies were RCTs; eight studies were performed in Asia, (24–28,31,32) three in Europe (13,16,22), two in Africa (15,29), and one in America (23). Participant ages ranged from 17–87 and the follow up periods were 1–6 weeks. Six trials (15,26,27,29,31,32) assessed the IPSS storage symptoms sub-scores, five trials (15,26,27,29,32) employed voiding symptoms sub-scores, and seven trials (13,16,22–25,30) evaluated USSQ urinary symptoms scores in order to evaluate the effectiveness of α1-adrenoceptor antagonists as therapeutic agents for patients with USS. VAPS was adopted in nine studies (13,15,16,23,25,26,28,30,32) and USSQ pain score was applied in six trials (13,16,22–25). The mean age of participants in each study was similar. In order to reduce any uncomfortable symptoms during the stenting periods, 0.4 mg q.d. tamsulosin, 2 mg q.d. terazosin, 2 mg b.i.d. terazosin, 10 mg q.d. alfuzosin or 4 mg q.d. doxazosin were administered as intervening measures. In the respective control groups of the included studies, either a placebo or a lack of α1-blockers treatment was employed. In eight studies, analgesics and/or antibiotics were used in all patients as a general therapy. No significant differences in the rates of employment and sexual activity were demonstrated between the treatment groups. The characteristics of the stones and ureteral stents in the treatment and control groups were outlined in Table II. The mean diameter of the stones in all of the groups was ~10 mm, and no significant differences in stone size were detected. The major indications of stent insertion were ureteroscopy, URS and ESWL. The ureteral stents used were composed of soft biomaterials, including polyurethane, with sizes ranging from 4.7–7 Fr.
Table I.
Basic features of included studies.
| Authors, year | Country | Sample size, T/Con (n) | Mean age, T/Con (years) | Intervention, dose | Control | Follow-up | Outcomes (weeks) | (Ref) |
|---|---|---|---|---|---|---|---|---|
| Dellis et al, 2014 | Greece | 50/50 | 45.6/46.9 | Tamsulosin, 0.4 mg qd | Placebo | 4 | A, Aq | (13) |
| Shalaby et al, 2013 | Egypt | 82/81 | 41.3/44.0 | Tamsulosin, 0.4 mg qd | Blank | 2 | B, C, D, H | (15) |
| Deliveliotis et al, 2006 | Greece | 50/50 | 53.1/55.3 | Alfuzosin, 10 mg qd | Placebo | 4 | A, Aq, D | (16) |
| Damiano et al, 2008 | Italy | 38/37 | NA/NA | Tamsulosin, 0.4 mg qd | Blank | 4 | Aa, Ab, Af, E, F | (22) |
| Beddingfield et al, 2009 | USA | 26/29 | 45.8/44.0 | Alfuzosin, 10 mg qd | Placebo | 1.5 | A, D | (23) |
| Park et al, 2009 | Korea | 20/12 | 47.7/54.4 | Alfuzosin, 10 mg qd | Placebo | 6 | Aa-e | (24) |
| Wang et al, 2009 | Taiwan | 79/75 | 50.1/51.5 | Tamsulosin, 0.4 mg qd | Placebo | 4 | A | (25) |
| Lee et al, 2010 | Korea | 15/18 | 43.3/45 | Tamsulosin, 0.2 mg qd | Blank | 1 | B, C, D | (26) |
| Navanimitkul et al, 2010 | Thailand | 21/21 | 46.1/51.5 | Tamsulosin, 0.4 mg qd | Blank | 4 | B, C, G | (27) |
| Mokhtari et al, 2011 | Iran | 37/36 | 45.03/41 | Terazosin, 2 mg qd | Placebo | 4 | B, D | (28) |
| Elnashar et al, 2011 | Egypt | 69/67 | 35/29 | Tamsulosin, 0.4 mg qd | Blank | 4 | B, C, G | (29) |
| Nazim et al, 2012 | Pakistan | 65/65 | 37.77/40.57 | Alfuzosin, 10 mg qd | Placebo | 1 | Aa, Aq, D | (30) |
| Kuyumcuoglu et al, 2012 | Turkey | 21/21 | 45.21/42.94 | Doksazosin, 4 mg qd | Blank | NA | B, C, D | (31) |
| Tehranchi et al, 2013 | Iran | 23/24 | 38.35/33.37 | Terazosin, 2 mg bid | Placebo | NA | B, C, D | (32) |
A, ureteral stent symptom questionnaire (USSQ) (six sections); Aa, USSQ urinary symptoms score; Ab, USSQ pain score; Ac, USSQ general health score; Ad, USSQ sexual matters score; Ae, USSQ work performance score; Af, USSQ additional problems score; Aq, USSQ quality of life score; B, international prostate symptom score (IPSS); C, IPSS quality of life score; D, visual analog pain scale (VAPS1–10); E, European quality of life 5-dimension questionnaire (EQ-5D); F, visual analog scale (0–100) in EQ; G, short-form 36 health survey scale (SF-36); H, overactive bladder questionnaire (OAB-q); T, treatmeant group; Con, control group; NA, not available; qd, quaque die; bid, bis in die.
Table II.
Uretal stone and stent characteristics in the treatment and control groups.
| Authors, year | Mean stone sizes, T/C (mm) | Stone location, T/C(%) | Stent indications, T/C(%) | Stent type | (Ref) |
|---|---|---|---|---|---|
| Dellis et al, 2014 | <10/<10 | LFU (54/48), RU (46/52) | ESWL (78/72), URS (22/28) | Percuflexplus, 6Frx24 or 6Frx26 | (13) |
| Shalaby et al, 2013 | NA | NA | URS/URL (69.5/69.1), PCNL (12.2/8.6), ESWL (17.1/19.8), EDL (1.2/2.5) | Polyurethane | (15) |
| Deliveliotis et al, 2006 | 7.6/7.1 | UU (18/20), MU (22/24), LU (60/56) | SEC, ESWL, URS | Polyurethane, size 5/26 or 5/28 | (16) |
| Damiano et al, 2008 | NA | NA | URS | Polyurethane, 7Fr | (22) |
| Beddingfield et al, 2009 | 6.35/7.21 | Ureteral and/or intrarenal | Ureteral and/or intrarenal urolithiasis | NA | (23) |
| Park et al, 2009 | NA | RU (40/41.7) LFU (60/58.3) | URS, PCNL, LPP, endoureterotomy | Polyurethane, 6Fr | (24) |
| Wang et al, 2009 | 9.0/9.4 | UU (10.1/10.7), MU (31.6/32), LU (58.2/57.3) | URS | Silicone coated, 7Fr | (25) |
| Lee et al, 2010 | 7.27/7.17 | UU (13.3/11.1), MU (26.7/27.8), LU (60/61.1) | URS | Percuflex, 6Fr | (26) |
| Navanimitkul et al, 2010 | NA | NA | URS (19/57.1), PCNL (42.9/19), Balloon dilatation (14.3/23.8) | Polyurethane, 6Fr | (27) |
| Mokhtari et al, 2011 | >10 but <15/>10 but <15 | NA | URS | Rosch, 4.8Fr | (28) |
| Elnashar et al, 2011 | NA | UU (37.7/70.1), LU (62.3/29.9) | URS | 6Frx26 | (29) |
| Nazim et al, 2012 | NA | UU (56.9/38.5), MU (18.5/24.6), LU (24.6/36.9) | URS | Polyurethane, 6Fr or 4.7Fr | (30) |
| Kuyumcuoglu et al, 2012 | 8.0/8.3 | NA | ESWL, URS | Polyurethane, 4.7Frx26 or 4.7Frx28 | (31) |
| Tehranchi et al, 2013 | 9.87/10.41 | UU (17.4/16.7), MU (17.4/8.3), LU (47.8/62.5), IR (17.4/12.5) | URS (82.6/87.5), PCNL (17.4/12.5) | 4.8 Frx28 | (32) |
ESWL, extracorporeal shock wave lithotripsy; PCNL, percutaneous nephrolithotomy; URS, ureteroscopy or ureteroscopy lithotripsy; URL, ureterolithotomy; SEC, spontaneous expulsion of the calculus; EDL, endopyelotomy; LPP, laparoscopicpyeloplasty; UU, upper ureter; MU, middle ureter; LU, lower ureter; RU, right ureter; LFU, left ureter; IR, intrarenal; T, treatment group; C, control group; NA, not available.
Assessing risk of bias
In terms of random sequence generation and selective reporting, all 14 studies were deemed ‘low’ risk (Table III); whereas the blinding of outcome assessment and other bias parameters were deemed ‘unclear risk’. Higher risk rates were demonstrated in the blinding of participants and personnel (42.86%) and allocation concealment (21.43%) parameters.
Table III.
Quality criteria and risk of bias.
| Number (%)a,b |
|||
|---|---|---|---|
| Items | High risk | Low risk | Unclear risk |
| Random sequence generation | 0 (0.00) | 14 (100.00) | 0 (0.00) |
| Allocation concealment | 3 (21.43) | 1 (7.14) | 10 (71.43) |
| Blinding of participants and personnel | 6 (42.86) | 7 (50.00) | 1 (7.14) |
| Blinding of outcome assesment | 0 (0.00) | 0 (0.00) | 14 (100.00) |
| Incomplete outcome data | 1 (7.12) | 13 (92.86) | 0 (0.00) |
| Selective reporting | 0 (0.00) | 14 (100.00) | 0 (0.00) |
| Other bias | 0 (0.00) | 0 (0.00) | 14 (100.00) |
Based on 14 randomized controlled trials that met the inclusion criteria.
Assessments made using the Cochrane risk of bias tool (version, 2011).
Urinary tract symptoms
USSQ and IPSS urinary symptoms scores were used to assess the patients' urinary tract symptoms during stenting periods. IPSS scores for urinary tract symptoms were divided into voiding and storage symptoms sub-scores, which were respectively pooled in the present meta-analysis. Voiding and storage symptoms data were available in five (15,26,27,29,32) and six studies (15,26,27,29,31,32), respectively. In terms of the results of statistical analysis, significant differences in IPSS voiding symptom sub-scores (MD, −2.66; 95% CI, −4.36 to −0.96) were detected; however, no significant differences in IPSS storage symptom sub-scores (MD, −0.93; 95% CI, −2.28 to 0.43) were detected (Table IV). The pooled USSQ urinary symptoms score data (MD, −5.84; 95% CI, −9.35 to-2.33) for seven studies (13,16,22–25,30) demonstrated that α1-blockers administration significantly reduced USS.
Table IV.
Meta-analysis results.
| Parameter | Outcomes | No. studies (Refs) | MD (95% CI) | Pooled I2 (%) | Model | Z-value | P-value | Favors α1-blockers |
|---|---|---|---|---|---|---|---|---|
| Urinary tract | IPSS storage sympyom sub-score | 6 (15,26,27,29,31,32) | −0.93 (−2.28, 0.43) | 73 | R | 1.34 | 0.18 | N |
| IPSS voiding symptom sub-score | 5 (15,26,27,29,32) | −2.66 (−4.36, −0.96) | 60 | R | 3.06 | 0.02 | Y | |
| USSQ urinary symptoms score | 7 (13,16,22–25,30) | −5.84 (−9.35, −2.33) | 92 | R | 3.26 | 0.001 | Y | |
| Pain | Visual analog pain scale (1–10) | 9 (13,15,16,23,25,26,28,30,32) | −1.53 (−2.25, −0.80) | 1 | F | 5.02 | <0.0001 | Y |
| USSQ pain score | 6 (13,23,25,22,16,24) | −3.97 (−5.52, −2.42) | 93 | R | 4.13 | <0.00001 | Y | |
| SF-36 pain score | 2 (27,29) | 0.35 (−0.04, 0.74) | 74 | F | 1.75 | 0.08 | Y | |
| Quality of life | IPSS quality of life score | 7 (15,25–27,29,31,32) | −1.46 (−2.64, −0.28) | 97 | R | 2.42 | 0.02 | Y |
| USSQ quality of life score | 3 (13,16,30) | −0.69 (−1.10, −0.28) | 0 | F | 3.32 | 0.0009 | Y | |
| Others | USSQ general health score | 5 (13,23,25,16,24), | −1.82 (−2.47, −1.18) | 39 | R | 5.53 | <0.00001 | Y |
| USSQ sexual matters score | 5 (13,23,25,16,24) | −0.10 (−0.79, 0.59) | 87 | R | 0.29 | 0.77 | N | |
| USSQ work performance score | 4 (13,23,25,24) | 1.64 (−2.18, 5.47) | 93 | R | 0.84 | 0.40 | N | |
| USSQ additional problems score | 3 (23,25,22) | −2.02 (−4.55, 0.52) | 78 | R | 1.56 | 0.12 | N | |
| Overactive Bladder Questionnaire | 2 (15,31) | −3.20 (−7.98, 1.58) | 98 | R | 1.31 | 0.19 | N |
USSQ, urinary symptoms score; IPSS, international prostate symptom score; SF-36, short-form 36 health survey scale; MD, mean difference; CI, confidence interval; R, random-effect model; F, fixed-effect model; Y, yes; N, no. All pooled results were from the estimate value of meta-analysis generated using Review Manager (version 5.2).
Pain
In order to evaluate the intensity of pain experienced by patients with an indwelling ureteral stent in the included studies, three approaches, the USSQ, VAPS and SF-36 pain scores, were used. VAPS data from nine studies (13,15,16,23,25,26,28,30,32) were pooled and a random effects model demonstrated a significant difference between the α1-blocker and control groups (MD, −1.53; 95% CI, −2.25 to −0.80) (Table IV); however, no significant differences were detected following sensitivity analysis. Similarly, USSQ pain scores (MD, −3.97; 95% CI, −5.52 to −2.42) (Table IV) were significantly decreased in patients treated with α1-blockers. No significant differences in the pooled SF-36 pain scores (MD, 0.35; 95% CI, −0.04 to 0.74) were detected in two studies (Table IV).
Quality of life
In order to assess the quality of life of patients following the placement of an indwelling ureteral stent, the IPSS scores in seven studies (15,25–27,29,31,32) and the USSQ scores in three studies (13,16,30) were pooled. In the α1-blockers groups, the IPSS (MD, −1.46; 95% CI, −2.64 to −0.28) and USSQ quality of life scores (MD, −0.69; 95% CI, −1.10 to −0.28) were significantly different, as compared with in the control groups (Table IV).
Other outcomes
In five studies (13,23,25,16,24), the effect size of the USSQ general health score (MD, −1.82; 95% CI, −2.47 to −1.18) suggested that additional health problems associated with the placement of ureteral stents improved following treatment with α1-blockers (Table IV). However, no significant differences were detected in the USSQ additional problems scores (MD, −2.02; 95% CI, −4.55 to 0.52) (22,23,25), the USSQ sexual matters scores (13,16,23–25) (MD, −0.10; 95% CI, −0.79 to 0.59), and the USSQ work performance scores (13,23–25) (MD, 1.64; 95% CI, −2.18 to 5.47) (Table IV). Furthermore, the OABq was used to assess whether irritative urinary symptoms had improved. No significant differences were demonstrated when the OABq scores from the two studies (15,31) were pooled [MD, −3.20, 95% CI (−7.98, 1.58)] (Table IV); however, a significant difference (P<0.001) was demonstrated between the α1-blocker treatment and control groups in a large, high quality study conducted by Shalaby et al (15).
Adverse events
According to the available data in the included studies, dizziness, headache and hypotension were the predominant side effects of treatment with α1-blockers. These side effects had little effect on the process of these trials (data not shown).
Discussion
The present systematic review and meta-analysis did not restrict the outcome measurements used in the included studies, and all methods of measures were included in the review. As compared with previous meta-analyses conducted by Yakoubi et al (33) and Lamb et al (34), numerous studies meeting the inclusion criteria have emerged in recent years, and the targets observed for USSQ have altered (15,27–29,31,32). Joshi et al (5) performed a study including 309 patients in order to broadly collate these symptoms and the USSQ was developed, covering a total of six sections and 38 scoring items, including: Urinary symptoms, pain, general health, work performance, sexual matters and additional problems (5). The urinary symptoms section includes 11 items assessing storage and voiding symptoms, incontinence, hematuria and dysuria; whereas the pain section includes eight scoring items evaluating various dimensions of pain. Although Deliveliotis et al (16) used the USSQ in a study investigating α1-blockers for the reduction of USS, not all patients suffer these symptoms, and another simple and effective questionnaire, known as the IPSS, has been widely used for assessing lower urinary symptoms in patients with benign prostatic hyperplasia (BPH) in previous trials. The IPSS is divided into voiding and storage symptoms sub-scores. Voiding symptoms include weak stream, hesitancy and intermittency; whereas storage symptoms include nocturia, daytime frequency and general urgency. Therefore, the IPSS may offer a simple and effective way to assess lower urinary tract symptoms associated with the placement of ureteral stents. Furthermore, pain is a common symptom experienced by patients with an indwelling ureteral stent (5) and VAPS has been widely-used as a tool to quantify the degree of pain, usually in combination with USSQ or IPSS when it is used to evaluate USS (14,15). In addition, the USSQ and IPSS quality of life scores were used to assess how USS affected the daily life of patients in the included studies.
In the present review, 14 studies, including a total of 1,075 patients and 14 outcome measures were selected for evaluation and analysis. The Cochrane Risk of Bias tool was used to assess the quality of each study, none of which were determined to be ‘very low’ quality. Urinary tract symptoms, pain and quality of life were the most common evaluation indices of USS. The findings of the present meta-analysis indicated that treatment with α1-blockers may relieve USS, based on the analysis of USSQ and IPSS urinary symptoms scores. Similarly, in all the included studies the USSQ, VAPS and SF-36 pain scores demonstrated that pain was significantly relieved in patients suffering from USS following treatment with α1-blockers. Furthermore, according to the outcome measures of the included studies evaluated in the present meta-analysis, the quality of life and general health of patients with USS improved following α1-blocker administration. However, no significant alterations in sexual activity, work performance, additional problems and OABq scores were detected. The predominant side effects of treatment with α1-blockers exhibited in the trials were dizziness, headaches and hypotension; however, these adverse events were rare. Therefore, we hypothesize that it may be safe to use α1-blockers to reduce USS.
Subgroup analyses were not conducted in the present meta-analysis; however, a sensitivity analysis was performed in order to investigate sources of heterogeneity. The meta-analysis result for the IPSS storage symptoms sub-score was significantly altered (MD, −1.46; 95% CI, −2.83 to −0.10) when the study conducted by Kuyumcuoglu et al (31) was removed; however this excluded study was low quality, with a small sample size and no placebo control, thus the results were unreliable. Similarly, the IPSS voiding symptom sub-scores were also altered when the studies conducted by Essam (MD, −2.92; 95% CI, −5.91 to 0.07) and Navanimitkul and Lojanapiwat (27) (MD, −1.04; 95% CI, −3.95 to 0.05) were removed. Following the exclusion of the small sample study conducted by Park et al (24) no significant differences or between-trial heterogeneity was detected in the USSQ sexually active scores following statistical analysis (MD, −0.10; 95% CI, −0.79 to 0.59). When the studies conducted by Elnashar and Shelbaia (29), and Nazim et al (30) were removed and the respective IPSS and USSQ quality of life scores were pooled, the results were significantly altered (MD, −1.20; 95% CI, −2.44 to 0.05 and MD, −0.28; 95% CI, −1.68 to 1.12, respectively). Furthermore, when the study conducted by Beddingfield et al (23) was excluded from the present meta-analysis, the results altered to favor α1-blockers administration (MD, −3.05; 95% CI, −4.40 to −1.70). With the exception of the aforementioned outcomes, outcomes were stable based on the results of the sensitivity analysis,.
Two previous meta-analyses by Yakoubi et al (four RCTs) and Lamb et al (five RCTs) have investigated the same topic and some of the major outcomes as the present meta-analysis, including urinary tract symptoms and pain (33,34). However, these meta-analyses had limitations, including: i) A single method (USSQ) was used to evaluate USS, therefore other questionnaires, including the IPSS, VAPS, SF-36 and OABq were not included; ii) an unreasonable method was used by Lamb et al to detect publication bias (<10 studies); iii) Lamb et al used the Jadad Scale to assess risk of bias, which lacks a consideration of allocation concealment and is not recommended for use by the Cochrane Collaboration (35); iv) no sensitivity analysis was performed and significant between-trial heterogeneity was detected; v) small sample sizes; vi) no adverse events were reported. In a study conducted by Mokhtari et al, the incidence of hematuria was similar between two groups; 57.6% in the terazosin group and 60.6% in the placebo group. Furthermore, as measured by IPSS urinal frequency, urgency and nocturia were significantly lower in the terazosin group, as compared with the placebo group (P<0.001) (28).
There were limitations in the present meta-analysis. Firstly, the various stent placement procedures and ureteral stent types may have had an effect on the evaluation of USS, including urinary symptoms and post-operative pain. Secondly, statistical analysis demonstrated 27
considerable between-trial heterogeneity. Either a placebo, the absence of α1-blocker administration or basic analgesics and antibiotics were used as controls in the included studies, which may have produced inconsistencies between the studies. Possible sources of between-trial heterogeneity include: Variable sample population, treatment duration, timing of outcome assessment, and additional analgesics or antibiotics; however, there is insufficient evidence to identify whether the heterogeneity existed as a result of the reasons stated. Thirdly, studies of low quality were included in the present meta-analysis, which may produce bias and reduce the reliability of the results. In addition, due to <10 studies ultimately being pooled, publication bias was undetectable and this bias may exist. Finally, the overall sample size of the present meta-analysis remained small.
In conclusion, the results of the present meta-analysis suggested that the use of α1-blockers beneficially influenced USS, particularly pain, urinary symptoms, quality of life and general health, in patients following a double-J ureteral stent. However, between-trial heterogeneity from the clinic and statistics was detected, which may have reduced the quality of the data. Large sample size, multicenter, well-designed RCTs are required in the future.
References
- 1.Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97:840–844. doi: 10.1016/S0022-5347(17)63130-6. [DOI] [PubMed] [Google Scholar]
- 2.Nabi G, Cook J, N’Dow J, McClinton S. Outcomes of stenting after uncomplicated ureteroscopy, Systematic review and meta-analysis. BMJ. 2007;334:572. doi: 10.1136/bmj.39119.595081.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pengfei S, Yutao L, Jie Y, Wuran W, Yi D, Hao Z, Jia W. The results of ureteral stenting after ureteroscopic lithotripsy for ureteral calculi, A systematic review and meta-analysis. J Urol. 2011;186:1904–1909. doi: 10.1016/j.juro.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 4.Al-Awadi KA, Halim Abdul H, Kehinde EO, Al-Tawheed A: Steinstrasse. A comparison of incidence with and without J stenting and the effect of J stenting on subsequent management. BJU Int. 1999;84:618–621. doi: 10.1046/j.1464-410x.1999.00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Joshi HB, Newns N, Stainthorpe A, MacDonagh RP, Keeley FX, Jr, Timoney Ureteral stent symptom questionnaire: Development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]
- 6.Keeley FX. Jr andT imoney AG: Routine stenting after ureteroscopy: Think again. Eur Urol. 2007;52:642–644. doi: 10.1016/j.eururo.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 7.Damiano R, Autorino R, Esposito C, Cantiello F, Sacco R, de Sio M, D’Armiento M. Stent positioning after ureteroscopy for urinary calculi: The question is still open. Eur Urol. 46:381–387. doi: 10.1016/j.eururo.2004.04.004. discussion 387-388, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Makarov DV, Trock BJ, Allaf ME, Matlaga BR. The effect of ureteral stent placement on post-ureteroscopy complications, A meta-analysis. Urology. 2008;71:796–800. doi: 10.1016/j.urology.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 9.Dellis A, Joshi HB, Timoney AG, Keeley FX., Jr Relief of stent related, symptoms, Review of engineering and pharmacological solutions. J Urol. 2010;184:1267–1272. doi: 10.1016/j.juro.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Lingeman JE, Preminger GM, Goldfischer ER, Krambeck AE. Comfort Study, Team, Assessing the impact of ureteral stent design on patient comfort. J Urol. 2009;181:2581–2587. doi: 10.1016/j.juro.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krambeck AE, Walsh RS, Denstedt JD, Preminger GM, Li J, Evans JC, Lingeman JE. Lexington Trial Study Group: A novel drug eluting ureteral, stent. A prospective, randomized, multicenter clinical trial to evaluate the safety and effectiveness of a ketorolac loaded ureteral stent. J Urol. 2010;183:1037–1042. doi: 10.1016/j.juro.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Tadros NN, Bland L, Legg E, Olyaei A, Conlin MJ. A single dose of a non-steroidal anti-inflammatory drug (NSAID) prevents severe pain after ureteric stent removal, A prospective, randomised, double-blind, placebo-controlled trial. BJU Int. 2013;111:101–105. doi: 10.1111/j.1464-410X.2012.11214.x. [DOI] [PubMed] [Google Scholar]
- 13.Dellis AE, Keeley FX, Jr, Manolas V, Skolarikos AA. Role of α-blockers in the treatment of stent-related symptoms: A prospective randomized control study. Urology. 2014;83:56–61. doi: 10.1016/j.urology.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 14.Kuehhas FE, Miernik A, Sharma V, Sevcenco S, Javadli E, Herwig R, Szarvas T, Schoenthaler M, Schatzl G, Weibl P. A prospective evaluation of pain associated with stone passage, stents, and stent removal using a visual analog scale. Urology. 2013;82:521–525. doi: 10.1016/j.urology.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Shalaby E, Ahmed AF, Maarouf A, Yahia I, Ali M, Ghobish A. Randomized controlled trial to compare the safety and efficacy of tamsulosin, solifenacin, and combination of both in treatment of double-j stent-related lower urinary symptoms. Adv Urol. 2013;2013:752382. doi: 10.1155/2013/752382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deliveliotis C, Chrisofos M, Gougousis E, Papatsoris A, Dellis A, Varkarakis IM. Is there a role for alpha1-blockers in treating double-J stent-related symptoms? Urology. 2006;67:35–39. doi: 10.1016/j.urology.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi: 10.1002/14651858.CD008509.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. J Clin Epidemiol. 2006;59:342–353. doi: 10.1016/j.jclinepi.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Lim KT, Kim YT, Lee TY, Park SY. Effects of tamsulosin, solifenacin, and combination therapy for the treatment of ureteral stent related discomforts. Korean J Urol. 2011;52:485–488. doi: 10.4111/kju.2011.52.7.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martov AG, Maksimov VA, Ergakov DV, Miroshnikov VM, Asfandiiarov FR, Kalashnikov ES. Tamsulosin administration for prophylaxis and treatment of stent-related symptoms. Urologiia. 2010:3–8. (In Russian) [PubMed] [Google Scholar]
- 22.Damiano R, Autorino R, De Sio M, Giacobbe A, Palumbo IM, D’Armiento M. Effect of tamsulosin in preventing ureteral stent-related morbidity A prospective study. J Endourol. 2008;22:651–656. doi: 10.1089/end.2007.0257. [DOI] [PubMed] [Google Scholar]
- 23.Beddingfield R, Pedro RN, Hinck B, Kreidberg C, Feia K, Monga M. Alfuzosin to relieve ureteral stent discomfort, A prospective, randomized, placebo controlled study. J Urol. 2009;181:170–176. doi: 10.1016/j.juro.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Park SC, Jung SW, Lee JW, Rim JS. The effects of tolterodine extended release and alfuzosin for the treatment of double-j stent-related symptoms. J Endourol. 2009;23:1913–1917. doi: 10.1089/end.2009.0173. [DOI] [PubMed] [Google Scholar]
- 25.Wang CJ, Huang SW, Chang CH. Effects of specific alpha-1A/1D blocker on lower urinary tract symptoms due to double-J stent, A prospectively randomized study. Urol Res. 2009;37:147–152. doi: 10.1007/s00240-009-0182-8. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Yoo C, Oh CY, Lee YS, Cho ST, Lee SH, Yang DY, Lee SK, Cho JS. Stent position is more important than α-blockers or anticholinergics for stent-related lower urinary tract symptoms after ureteroscopic ureterolithotomy, A prospective randomized study. Korean J Urol. 2010;51:636–641. doi: 10.4111/kju.2010.51.9.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navanimitkul N, Lojanapiwat B. Efficacy of tamsulosin 0.4 mg/day in relieving double-J stent-related symptoms: A randomized controlled study. J Int Med Res. 2010;38:1436–1441. doi: 10.1177/147323001003800425. [DOI] [PubMed] [Google Scholar]
- 28.Mokhtari G, Shakiba M, Ghodsi S, Farzan A. HeidariN ejad S and Esmaeili S: Effect of terazosin on lower urinary tract symptoms and pain due to double-J stent: A double-blind placebo-controlled randomized clinical trial. Urol Int. 2011;87:19–22. doi: 10.1159/000323855. [DOI] [PubMed] [Google Scholar]
- 29.Elnashar A, Shelbaia A. Role of tamsulosin in improving double-j ureteric stent-related symptoms. Afr J Urol. 2011;17:111–114. [Google Scholar]
- 30.Nazim SM, Ather MH. Alpha-blockers impact stent-related symptoms: A randomized double-blind, placebo-controlled trial. J Endourol. 2012;26:1237–1241. doi: 10.1089/end.2012.0036. [DOI] [PubMed] [Google Scholar]
- 31.Kuyumcuoglu U, Eryildirim B, Tuncer M, Faydaci G, Tarhan F, Ozgül A. Effectiveness of medical treatment in overcoming the ureteral double-J stent related symptoms. Can Urol Assoc J. 2012;6:E234–E237. doi: 10.5489/cuaj.10143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tehranchi A, Rezaei Y, Khalkhali H, Rezaei M. Effects of terazosin and tolterodine on ureteral stent related symptoms, A double-blind placebo-controlled randomized clinical trial. Int Braz J Urol. 2013;39:832–840. doi: 10.1590/S1677-5538.IBJU.2013.06.09. [DOI] [PubMed] [Google Scholar]
- 33.Yakoubi R, Lemdani M, Monga M, Villers A, Koenig P. Is there a role for α-blockers in ureteral stent related symptoms? A systematic review and meta-analysis. J Urol. 2011;186:928–934. doi: 10.1016/j.juro.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 34.Lamb AD, Vowler SL, Johnston R, Dunn N, Wiseman OJ. Meta-analysis showing the beneficial effect of α-blockers on ureteric stent discomfort. BJU Int. 2011;108:1894–1902. doi: 10.1111/j.1464-410X.2011.10170.x. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. (oct18 2) [DOI] [PMC free article] [PubMed] [Google Scholar]