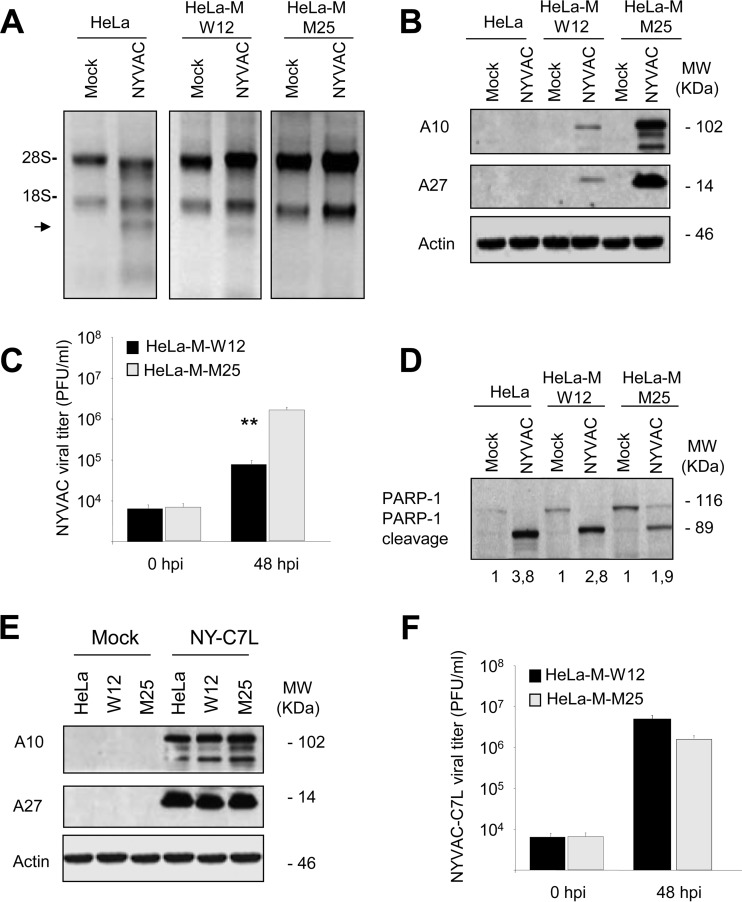
FIG 4.
RNA degradation, protein synthesis, virus growth, and apoptosis induction in HeLa, HeLa-M-W12, or HeLa-M-M25 cells infected with NYVAC. (A) HeLa, HeLa-M-W12, and HeLa-M-M25 cells were mock infected (M) or infected with NYVAC (5 PFU/cell). Total rRNA was isolated from cells uninfected or infected with NYVAC at 16 hpi; 2 μg of each sample was applied for electrophoresis, and the gel was stained with ethidium bromide. (B) Viral protein expression during NYVAC infection. HeLa, HeLa-M-W12, and HeLa-M-M25 cells were mock infected (M) or infected with NYVAC (5 PFU/cell). Cells were harvested at 16 hpi, and equal amounts of proteins from cell extracts were fractionated by SDS-PAGE, transferred to nitrocellulose, and treated with specific antibodies to A10 or A27 viral proteins. Actin was used as a loading control. Molecular masses (“MW”; in kilodaltons) are indicated and are based on protein standards. (C) Cells were infected, and at the different times indicated cells were harvested and virus yields were determined by plaque assay. Results represent the means ± the standard deviations of three independent experiments. P values from a two-tailed t test assuming nonequal variance were determined. In all cases, we obtained P values of <0.01. (D) PARP-1 cleavage during NYVAC infection. HeLa, HeLa-M-W12, and HeLa-M-M25 cells were mock infected (M) or infected with NYVAC (5 PFU/cell). At 16 hpi, cells were harvested and equal amounts of proteins from cell extracts were fractionated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-PARP-1 antibodies. Numbers appearing under each lane represent the ratio of the intensity of the band corresponding to the PARP-1 cleaved fragments in infected cells to those levels in uninfected cells, as determined by densitometric analysis. Molecular masses (“MW”; in kilodaltons) are indicated and are based on protein standards. (E) HeLa, HeLa-M-W12, and HeLa-M-M25 cells were mock infected (M) or infected with NYVAC-C7L (5 PFU/cell). At 16 hpi, cells were collected and equal amounts of proteins from cell extracts were fractionated by SDS-PAGE, transferred to nitrocellulose, and treated with specific antibodies to A10 or A27 viral proteins. Actin was used as a loading control. (F) HeLa-M-W12 and HeLa-M-M25 cells were infected with NYVAC-C7L, and at the different times indicated cells were harvested and virus yields were determined by plaque assay. Results represent the means ± the standard deviations of three independent experiments. P values from a two-tailed t test assuming nonequal variance were determined. In all cases, we obtained P values of <0.01.