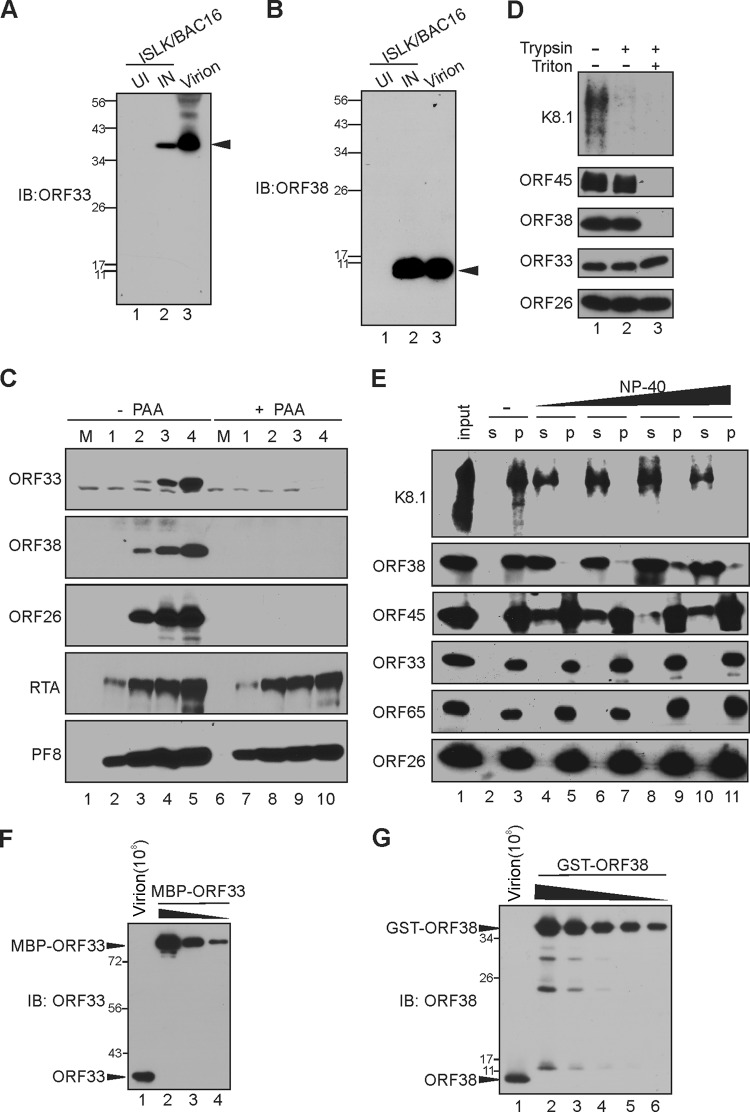
FIG 1.
Characterization of KSHV ORF33 and ORF38. (A and B) Generation of anti-ORF33 and anti-ORF38 monoclonal antibodies. iSLK carrying BAC16 was left untreated or was induced with doxycycline and sodium butyrate for 3 days. The uninduced (UI) or induced (IN) cell lysates, as well as extracellular virions, were resolved by SDS-PAGE and then analyzed by Western blotting using anti-ORF33 (A) or anti-ORF38 (B) antibody. The black arrows indicate ORF33 (A) or ORF38 (B). IB, immunoblot. (C) ORF33 and ORF38 express with true late kinetics. iSLK cells carrying BAC16 were induced with doxycycline and sodium butyrate in the presence or absence of phosphonoacetic acid (PAA), and the cell lysates were harvested at the indicated day postinduction (dpi). Total proteins were resolved by SDS-PAGE and detected with the indicated antibodies. M, molecular size marker. (D) Differential sensitivity of ORF33 and ORF38 to trypsin digestion. The extracellular virions were concentrated and purified as outlined in Materials and Methods. The purified virions were treated with Triton X-100, trypsin, or both. Virion envelope, tegument, and capsid proteins were analyzed by Western blotting with the indicated antibodies. (E) Sensitivities of ORF33 and ORF38 to detergent treatment. Purified virions were treated with increasing concentrations of NP-40, after which the virions were pelleted down through a 25% sucrose cushion. The virion proteins in the supernatant (s) or pellet (p) were detected with the indicated antibodies. (F and G) The molecular numbers of ORF33 and ORF38 are comparable in the viral particle. Purified virions (108) and serially diluted GST-ORF38 and MBP-ORF33 proteins were resolved by SDS-PAGE and then analyzed by Western blotting with the indicated antibodies. The blot signals were plotted with ImageJ, and ORF33 and ORF38 copy numbers were determined by using GST-ORF38 or MBP-ORF33 as standards. Values stated in the text are representative of technical duplicates.