FIG 2.
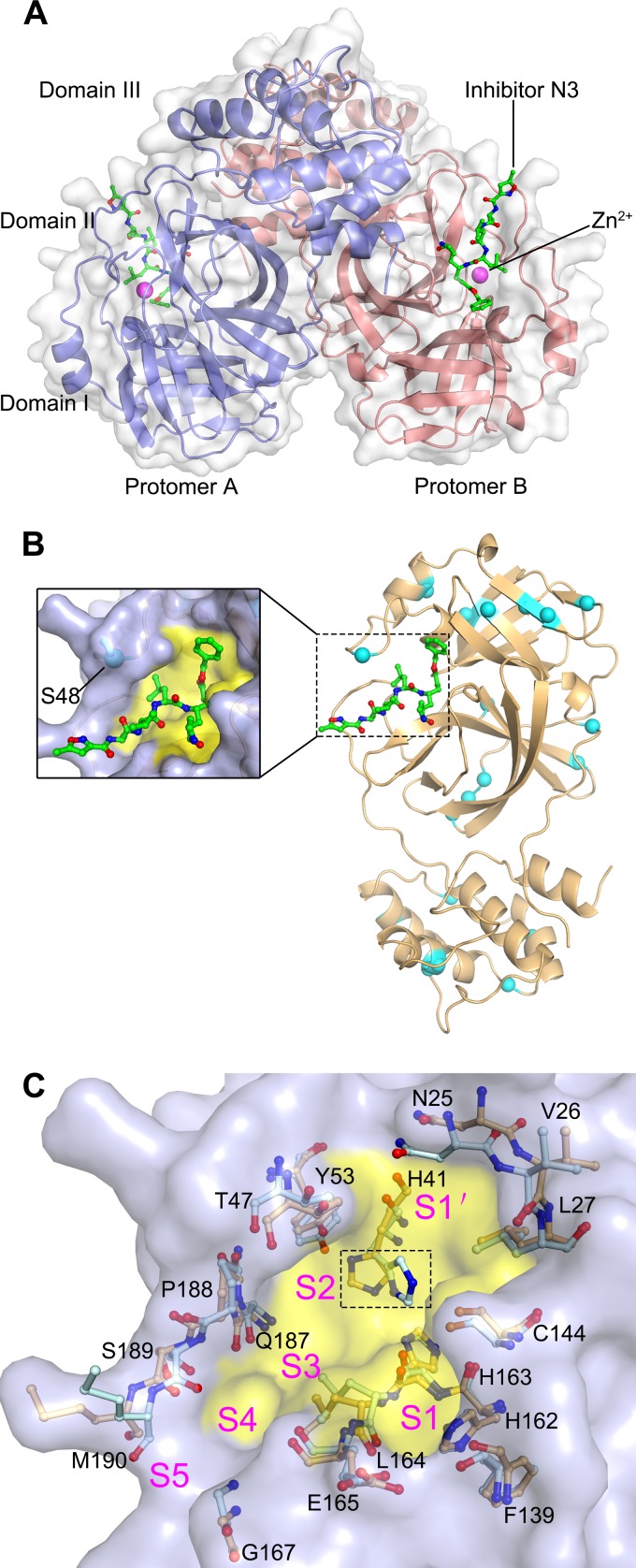
The structure of FIPV Mpro. (A) Overall structure of the FIPV Mpro-Zn2+-N3 complex in surface representation. The two protomers are colored slate and deep salmon, inhibitor N3 is shown as green sticks, and Zn2+ is shown as a magenta sphere. (B) Distribution of the nonconserved residues between FIPV and TGEV Mpros. The structure of FIPV Mpro is shown in cartoon representation (light orange). The nonconserved residues between FIPV Mpro and TGEV Mpro are shown as cyan spheres. The N3 molecule is shown as green sticks, and the substrate-binding pocket is colored in yellow. A zoomed view of the substrate-binding pocket is shown to the left. (C) Superimposition of the substrate-binding pocket of FIPV Mpro-Zn2+-N3 (light orange) with that of TGEV Mpro (pale cyan). The substrate-binding pocket of the FIPV Mpro complex is colored in yellow. The S1, S2, S4, S5, and S′ subsites are labeled. The key amino acids constituting the substrate-binding pockets of FIPV Mpro and TGEV Mpro are shown as sticks. The His41 residues specifically are marked by the dashed rectangular box.