Abstract
Dementias are among the most common neurological disorders, and Alzheimer’s disease (AD) is the most common cause of dementia worldwide. AD remains a looming health crisis despite great efforts to learn the mechanisms surrounding the neuron dysfunction and neurodegeneration that accompanies AD primarily in the medial temporal lobe. In addition to AD, a group of diseases known as frontotemporal dementias (FTDs) are degenerative diseases involving atrophy and degeneration in the frontal and temporal lobe regions. Importantly, AD and a number of FTDs are collectively known as tauopathies due to the abundant accumulation of pathological tau inclusions in the brain. The precise role tau plays in disease pathogenesis remains an area of strong research focus. A critical component to effectively study any human disease is the availability of models that recapitulate key features of the disease. Accordingly, a number of animal models are currently being pursued to fill the current gaps in our knowledge of the causes of dementias and to develop effective therapeutics. Recent developments in gene therapy-based approaches, particularly in recombinant adeno-associated viruses (rAAVs), have provided new tools to study AD and other related neurodegenerative disorders. Additionally, gene therapy approaches have emerged as an intriguing possibility for treating these diseases in humans. This chapter explores the current state of rAAV models of AD and other dementias, discuss recent efforts to improve these models, and describe current and future possibilities in the use of rAAVs and other viruses in treatments of disease.
Keywords: Tau protein, Neurofibrillary tangle, Recombinant adeno-associated virus, Hippocampus, Entorhinal cortex, Animal model
1 Alzheimer’s Disease and Other Dementias
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder involving progressive degeneration in regions of the brain that are important for memory and cognition (i.e., the temporal lobe and hippocampus). The neuropathological hallmarks of AD include accumulations of the amyloid-β peptide (Aβ) and the tau protein. The Aβ pathology is in the form of extracellular plaques (i.e., senile plaques), while the tau pathology is intracellular inclusions known as neurofibrillary tangles (NFTs), neuropil threads, and neuritic plaques [1, 2]. The presence of Aβ plaques and tau NFTs are required for a diagnosis of AD; however, the role each pathology plays in the mechanisms of the disease and what initiates their production remain unknown. The majority of AD cases are sporadic in nature but approximately 5 % are due to a specific genetic polymorphism in the amyloid precursor protein gene (APP) or one of the presenilin genes (PS1 or PS2) that make up part of the APP-cleaving complex [3, 4]. Familial AD typically presents with pathology similar to sporadic AD, but with an earlier onset and faster progression indicating that the mechanisms of disease may be similar but more aggressive in inherited cases.
The Aβ peptide was the focus of much of the AD research over the last several decades because the familial mutations are located in APP and its processing machinery. The peptide is the primary component of the extracellular plaques that were originally identified in AD brains [2]. The peptide is cleaved from APP, an integral membrane protein, by various secretase enzymes that determine the length of the peptide. The Aβ40 form (i.e., 40 amino acids long) is more prevalent but the Aβ42 form (i.e., 42 amino acids long) is more prone to aggregate and linked with toxicity [5, 6]. While amyloid plaque density does not correlate closely to progression of AD [7], recent research identified that the levels of soluble oligomers and the ratio of the Aβ42 to Aβ40 are more closely associated with disease-related toxicity [8, 9]. Currently, the role of Aβ in disease remains unclear, but Aβ may directly or indirectly induce neurodegenerative effects such as synaptic defects through interactions with receptors [10], activation of caspases [11], formation of pores [12], and/or axonal transport dysfunction through kinase dysregulation [13]. Many of the toxic effects associated with Aβ expression in animal and cell culture models require the presence of the tau protein, indicating a link between the toxicity of the peptide and the other major pathology associated with AD [14–16].
Indeed, a great deal of recent research has focused on tau pathology in AD and its role in the disease progression. Tau is a microtubule-associated protein that may be involved in stabilizing and spacing microtubules as well as regulating axonal transport, as well as kinases and phosphatases [17–20]. In the adult human central nervous system (CNS), six different isoforms of tau are created by alternative splicing of the 2nd, 3rd, and 10th exons. The 10th exon contains one of four potential microtubule-binding repeat regions that are necessary for microtubule association and contain the regions that make up the core of tau aggregates. Isoforms that contain all four microtubule-binding regions are referred to as 4R, while isoforms without exon 10 are known as 3R isoforms.
In AD, it is believed that tau dissociates from microtubules, begins to aggregate, and is mislocalized from axons to the somato-dendritic compartment [21]. Phosphorylation of tau is increased approximately fourfold and some forms of phosphorylated tau do not bind as well to microtubules and aggregate more readily than unmodified tau [22, 23]. Aβ appears to enhance tau aggregation, but not vice versa, in some mouse models that combine disease-related forms of APP and tau [24]. These studies suggest that Aβ may have an upstream role in the disease by altering tau dysfunction in some way [24]. In fact, tau was initially believed by some to be merely a byproduct of Aβ pathology until the discovery of inherited, early-onset FTDs that were caused by mutations in the tau gene [25]. This provided evidence that pathological changes in tau were sufficient to cause a neurodegenerative disease. Further evidence for a role of tau in neurotoxicity came with data suggesting that some of the toxic effects of Aβ appear to be dependent on the presence of tau [14–16]. Ultimately, the toxic effects of abnormal forms of tau are thought to lead to dysfunction and degeneration of synapses and axons that in turn lead to the loss of memory and cognitive deficits that characterizes AD [26, 27].
The progressive cognitive decline in AD correlates well with a stereotypical spatiotemporal distribution of tau pathology [1, 7]. The first stage is called Braak stage I, which is characterized by the initial appearance of tau pathology in the transentorhinal cortex of the temporal lobe. In Braak stage II, tau pathology expands into the entorhinal cortex (EC) and first appears in the CA1 neurons of the hippocampus (HP). Braak stage III is characterized by tau deposits in the subcortical regions of the thalamus and amygdala as well as throughout the entire EC. In Braak stage IV, large numbers of NFTs and neuropil threads are present in the EC along with an increasing number of NFTs in the CA1 and CA4 neurons and the striatum, amygdala, thalamus, and hypothalamus. Tau pathology expands throughout the HP in Braak stage V in conjunction with growing numbers of tau aggregates in the isocortex and subcortical regions. At Braak stage VI, large amounts of tau pathology is found in all regions of the neocortex and isocortex, subiculum, subcortical nuclei, thalamus, hypothalamus, and substantia nigra (SN). As the tau pathology progresses, specific populations of neurons begin to die, worsening the cognitive dysfunction and often leaving behind “ghost tangles” (starting around Stage III and increasing in number through Stage VI), which are the remains of tangles that become extracellular once a neuron dies [28].
NFTs were traditionally thought to be the toxic forms of tau but a number of studies suggest pre-tangle aggregates, such as oligomers, may be the toxic species. Neurons appear to survive for decades containing NFTs [29] and recent work showed that neurons containing NFT-like inclusions remain functionally integrated in the brain of a transgenic tau mouse [30]. Additionally, treatment of cells (i.e., cell lines and primary neurons) with preformed oligomers leads to toxicity in a number of model systems, while treatment with filaments does not [31–33]. Tau pathology appears in degenerating axons prior to the appearance of NFTs in the somatodendritic compartment [34] and the presence of NFTs are not harmful to neurons in the absence of continued tau expression in a transgenic mouse model [35].
In addition to AD, there are a number of less prevalent diseases that are characterized by pathological tau aggregations in the absence of amyloid pathology (reviewed in Ref. [36]). Progressive supranuclear palsy (PSP) is a disease presenting vertical gaze palsy and atypical parkinsonism due to degeneration in regions of the basal ganglia, brainstem, and cerebellum [37, 38]. Pick’s disease (PiD) is characterized by dementia and aphasia along with neuropathologic rounded tau aggregates, known as Pick bodies, in the cortex and limbic lobe [39]. Corticobasal degeneration (CBD), is associated with focal cortical degeneration leading to asymmetrical rigidity and apraxia [40]. Phosphorylated tau accumulates in neuronal processes and astrocytes primarily located in the cortex and basal ganglia. Chronic traumatic encephalopathy (CTE), a sporadic tauopathy believed to be the result of repeated head traumas, is associated with frontotemporal and HP atrophy along with varying forms of tau aggregates and symptoms including progressive dementia and behavioral changes [41]. FTD with Parkinsonism linked to Chromosome 17 (FTDP-17) are tauopathies caused by inherited mutations in the tau gene that lead to early-onset dementia and brain atrophy, typically occurring in the cortex, basal ganglia, and limbic lobe [25]. In FTDP-17, the tau pathology occurs in a variety of brain regions and can vary in type (i.e., NFTs, Pick bodies, etc.), and the clinical symptoms can include dementia, parkinsonism, and psychosis. Expression of tau containing FTDP-17 mutations is widely used to generate model systems for studying the protein’s role in disease due to their aggressive phenotypes [42, 43]. Many of the mutations are simple missense mutations that change a single amino acid or silent mutations that alter mRNA splicing, but these changes are sufficient to induce a neuro-degenerative disease. Interestingly, the tau pathology present in different tauopathies can vary in their composition of tau isoforms. For example, the tau pathology in AD and CTE contains all six of the tau isoforms [41], PSP and CBD display aggregates made up primarily of 4R tau isoforms, and PiD contains mainly 3R tau isoforms (reviewed in Ref. [44]).
Our understanding of the molecular mechanisms underlying the degeneration that occurs in AD and other tauopathies is unclear at this point. A number of proposed hypotheses exist, such as dysfunctional neurotransmission, oxidative damage, reduced cytoskeletal integrity, misregulation of important enzymes (e.g., kinases, phosphatases, proteases), protein degradation impairment, ion imbalances, axonal dysfunction (e.g., transport impairment) and inflammation (reviewed in Refs. [45–53]). Indeed, these diseases likely are an amalgamation of many dysfunctional processes and as such each of these avenues warrants further investigation. One of the most critical components of advancing the field and potentially developing treatments are the animal models that recapitulate key aspects of these diseases.
2 Transgenic Animal Models of AD and Tauopathies
Historically, the majority of in vivo models of AD and other tauopathies are transgenic mice engineered to express proteins related to the diseases and these models are reviewed elsewhere [54–56]. Many of these models attempt to recapitulate one or both of the major pathological hallmarks of the disease, Aβ plaques and tau NFTs, by overexpressing mutant proteins that are associated with familial forms of AD and FTDs. Some of the first mouse transgenic models used wild-type or single, double, or triple familial AD mutations in the APP gene to generate Aβ plaques. All of these models displayed amyloid plaques but with relatively limited cognitive impairment and no neuronal loss [57–61]. The trend toward models with more mutations continued with the addition of mutated forms of presenilin 1, a protein involved in the proteolytic processing of APP. A number of lines were generated by crossing mutant APP mice with mutant PS1 mice, and these crosses typically resulted in enhanced levels of the Aβ peptide as well as early development of plaques and cognitive deficits [62–65] but only the 5×FAD mouse that harbors 3 APP mutations and 2 PS1 mutations showed overt neurodegeneration [66]. Interestingly, when P301L tau transgene was added to mutant APP and PS1 to create the so-called triple transgenic line, there was progressive synapse loss, Aβ and tau pathology accumulation, as well as overt neurodegeneration [67]. A recent transgenic rat model further demonstrated a link between amyloid and tau pathologies and recapitulated several key aspects of AD. The TgF344-AD line included APPsw and PS1ΔE9 transgenes that resulted in age-dependent cerebral amyloidosis that precedes tau pathology and loss of HP and cortical neurons resulting in cognitive defects, which are effects not seen when these mutations are present in mice [68, 69]. One important distinction between mice and rats is that rats contain the full complement of all six tau CNS isoforms, while adult mice do not [70, 71]. Thus, based on the importance of tau for toxicity in these models, it is becoming more evident the tau protein plays a key role in these diseases.
Much like the APP and PS1 mouse models, a number of transgenic lines were generated using inherited FTD tau mutations in the transgenes [43, 72–74]. The initial transgenic mice that expressed wild-type human tau resulted in hyperphosphorylation of the protein but lacked the more mature NFT-like inclusions associated with tauopathies [75, 76]. One of most widely used tau mutations in animal modeling is P301L tau. The JNPL3 mice expressing P301L tau presented aging-related cognitive and motor defects that were associated with NFT-like inclusions in the amygdala, hypothalamus, midbrain, and septal nuclei, and loss of motor and spinal cord neurons [43]. P301L tau was also used in the rTg4510 transgenic line that allowed temporal regulation of transgene expression through a tetracycline-induction system [35]. Most recently, transgenic mice, with locally restricted expression of tau, were used to demonstrate propagation of tau pathology from the EC, demonstrating that trans-synaptic spread of tau could be a mechanism for the progression of tau pathology [77, 78]. The tau transgenic mice have helped move the field forward in important ways; however, there are a number of caveats that must be considered when using transgenic models.
3 Potential Caveats Associated with Transgenic Models
Transgenic mouse models have provided insight into mechanisms of AD, and related disorders, and continue to be useful tools. However, despite their popularity, there is a number of potential limitations that must be considered when using transgenic models to study diseases. First, the combination of genes used in some models makes it difficult to clearly determine what is driving the observed effects. It is worth noting that humans do not contain any of the combinations of gene mutations and, in some cases, the combinations include mutations from different diseases in the same mouse (e.g., AD and FTDP-17). Often, these models develop pathology in a number of brain regions that are largely unaffected in the human diseases and overt neurodegeneration occurs only when multiple genes are combined (except in a number of tau transgenic lines). The vast majority of transgene promoters used do not provide enough specificity to target expression in the select neuronal populations affected in the diseases, and the proteins are typically overexpressed. This limits the ability of the models to recapitulate the region-specific onset of pathology that characterizes the human diseases. The human diseases are adult-onset, with age as a primary risk factor for many of them, which is a difficult aspect for most transgenic mouse models to recapitulate because the mutated forms of human proteins are present in the germ-line cells and expressed constitutively. Lifelong expression could give rise to compensatory changes in the animals that make it difficult to extend results to sporadic adult-onset diseases. Finally, an important practical limitation of transgenic models is that they can take a long time to generate and often require significant financial input making them unsuitable for rapidly and inexpensively testing a number of transgenes.
Some of the problems associated with transgenic models are unavoidable in all model systems, and it must be acknowledged that models are capable of approximating only some aspects the diseases. However, the use of complementary model systems can help to address many of the limitations related to the transgenic approach. One such system uses viral vector technology to deliver genetic material to cells in a safe, controlled, and reproducible manner. Current gene therapy technology has opened new possibilities in AD and tauopathy research by providing tools to develop novel models of these diseases.
3.1 Viral Vector Delivery Systems
Viral vectors are a common vehicle to deliver genetic material in non-transgenic animal model systems (reviewed in Refs. [79, 80]). Here, we will focus on recombinant adeno-associated viruses (rAAVs), which are popular among the available viral vectors (comparisons of other viral vectors can be found in Chapter 1). These are small, single-stranded DNA viruses that require a helper virus, such as an adenovirus, for infection and are non-replicative. The viruses can transduce several cell types within the CNS including neurons, astrocytes, and oligodendrocytes depending on the specific viral serotype [81]. Briefly, viruses are internalized in a receptor-dependent fashion and after infection the DNA is transported to the nucleus. The genetic information is replicated to form double-stranded DNA and then transcribed to produce the gene of interest, which can last for extended periods of time (see Chapters 1 and 10 for more details on rAAV biology and transduction mechanisms). Importantly, the transduction specificity (i.e., cell-type selectivity), ability to inject into specific brain regions and at specific times in lifespan, long-term gene expression, and lack of eliciting a strong immunogenic response make rAAVs ideal for modeling neurodegenerative diseases. In addition to their potential in basic research, they also show promise as gene transfer therapeutics for neurodegenerative diseases in the CNS.
4 Advantages of Viral Vector Systems
Viral delivery systems hold a number of advantageous characteristics that are difficult to achieve with other approaches. Viral vector systems provide exquisite control over the temporal expression of the gene of interest. AD and other tauopathies are all adult-onset and aging remains the primary risk factor of developing AD. Thus, studies that introduce the production of disease-related genes of interest should incorporate this important variable by expressing the genes in adult or elderly animals. Delivery of viral vectors is completely under the control of the researcher, which easily facilitates studies where animals are transduced at any point in the lifespan. For example, injection of rAAV2/5-GFP and rAAV2/5-human wild-type tau (2N4R) into the HP of young adult (6 months) and old aged (20 months) Fischer 344 rats results in efficient neuronal transduction and similar levels of protein expression after 1 month (Fig. 1). Our group recently found that rAAV2/5-GFP transduction in the SN is reduced in aged animals compared to young animals [82], but other studies have shown that rAAV2/9-tau and -GFP transduction is unaffected in the SN [83, 84]. The differences in transduction efficiency with age may reflect the use of different rAAV serotypes. These studies suggest that transduction efficiency in aging animals differs in specific brain regions and with different rAAV serotypes. Virally transduced cells maintain expression of the protein without the addition of other molecules for the remainder of the lifespan. Much like inducible transgenic lines, rAAV-mediated expression can be further regulated if tetracycline regulatory elements are incorporated into the rAAV systems [85].
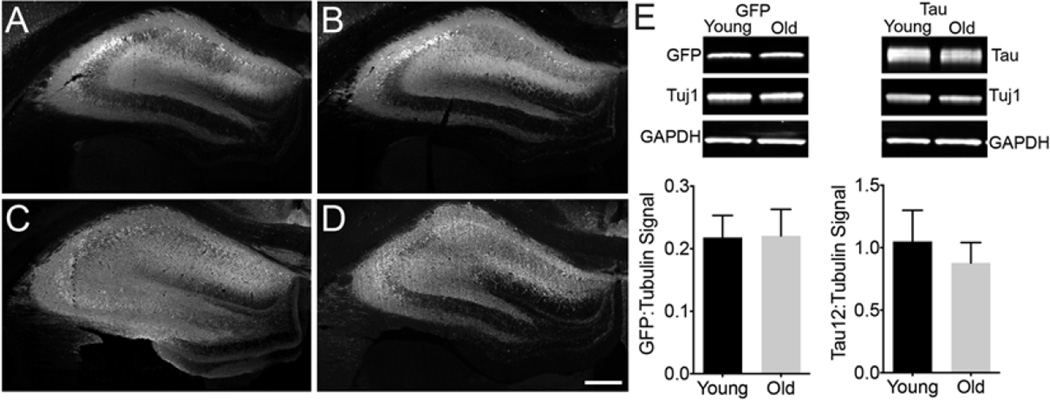
Fig. 1.
rAAV2/5 efficiently transduces neurons and produces equal protein expression in the young and aged rat hippocampus (HP). (a–d) Young adult (a and c, 6 months, n = 3) and old aged (b and d, 20 months, n = 3) Fischer 344 rats were injected with 2 µl (titer 1 × 1013 vg/ml, 0.3 µl/min infusion rate) rAAV-GFP (a and b) or rAAV-human tau (c and d) into the dorsal HP (-3.8 mm AP and +2 mm ML from bregma, and −2.6 mm DV from the dura) using pulled glass syringe tips attached to a Hamilton gastight syringe (#7653-01). In all conditions, rAAVs produced widespread transduction of hippocampal neurons. Endogenous GFP signal was used for imaging GFP injected animals, while Tau12 antibody (1:40,000; [144]), a human tau-specific monoclonal antibody, was used to label human tau in rAAV-tau injected animals. Our group has observed efficient transduction of all neuron types in the HP with rAAV2/5 (e.g., CA pyramidal neurons and dentate granule cells). Scale bar = 400 µm. (e) Lysates of the dorsal HP were run on a western blot to quantify the amount of rAAV-derived proteins (e.g., GFP and human tau, n = 3/group). Blots were probed with GFP antibody (Abcam, ab290, 1:3,000), Tau12 (1:200,000), and two loading controls βIII-tubulin antibody (Tuj1, 1:10,000; [145]) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Cell Signaling, 5174, 1:2,000). The signal intensity for each band was quantified using the Licor Image Studio software and signal intensities for GFP or tau are expressed as a ratio to tubulin signal intensities (similar results were obtained when normalized to GAPDH signal). No aging-related differences were observed with either GFP levels (unpaired t-test, p = 0.93) or human tau levels (unpaired t-test, p = 0.46). Immunofluorescence and Licor immunoblotting were performed similar to previously published methods [146]
In addition to great temporal control, viral vectors provide control over the spatial expression of the transduced genes. AD and other tauopathies are characterized by the degeneration and pathological accumulation of proteins in specific brain regions. For example, the EC and HP are primary affected areas in AD, while other tauopathies involve degeneration in the frontal and temporal cortices, as well as the basal ganglia, brainstem and cerebellum. Viral vectors allow researchers to stereotaxically inject viruses in relatively discrete brain regions of interest. For example, direct injection of rAAVs into the rat HP or EC results in efficient transduction of neurons (Fig. 2). Furthermore, unilateral injections allow the contralateral half of the brain to serve as a control within the same animal, but the contralateral projections of a specific region must be considered. Another level of specificity can be obtained by using cell-specific promoter systems [86, 87], which can also be used in transgenic models [78, 88]. Finally, the serotypes of AAVs exhibit significant differences in cell tropism allowing for increased specificity of transducing different cell types (e.g., neurons, glia, or both) and specific brain regions [89, 90].
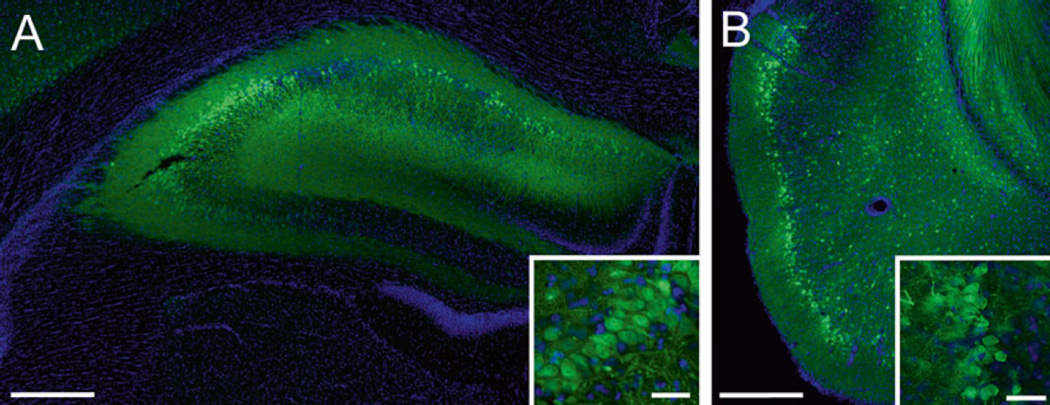
Fig. 2.
rAAV2/5 effectively transduces neurons in the hippocampus (HP) and entorhinal cortex (EC) of Fischer 344 rats. (a) A representative image of the dorsal HP of a young adult (6 months) rat transduced with rAAV-GFP (as in Fig. 1, scale bar = 400 µm). The inset illustrates GFP expressing CA3 pyramidal neurons in the HP (scale bar = 50 µm). (b) A representative image of the EC of a young (3 months) rat transduced with rAAV-GFP (titer = 1.9 × 1013, coordinates: −5.8 mm AP, +3.3 mm ML from bregma, −8.8 mm DV from dura, at 17° angle; scale bar = 400 µm). Note that layer II/III EC neurons are well transduced (inset, scale bar = 50 µm); these neurons are particularly susceptible in AD. Endogenous GFP fluorescence was used for imaging transduced cells
Additional advantages of viral vector systems are the relative ease and short timeframe (i.e., a few months) of generating new viral vectors as well as the low cost of production. Typically, the process involves simply cloning the gene of interest into the appropriate rAAV expression plasmid, and then, in the case of many investigators, sending the expression plasmids to a reputable gene therapy core facility for production. Other investigators choose to generate their own viral vectors ([91] and Chapters 7–9). Thus, viral vector models can be adjusted relatively quickly to test new variants of a particular protein or to examine interactions between proteins. Alternately, viruses can introduce RNA -based inhibitors of gene expression (e.g., shRNA or microRNAs) in a time- and region-specific manner for a relatively simple and selective approach to genetic knockdown [92]. This level of versatility lends itself well to creating numerous novel model systems and easily manipulating the genes/proteins of interest, which is difficult to achieve with other approaches.
5 Potential Caveats with Viral Vector Systems
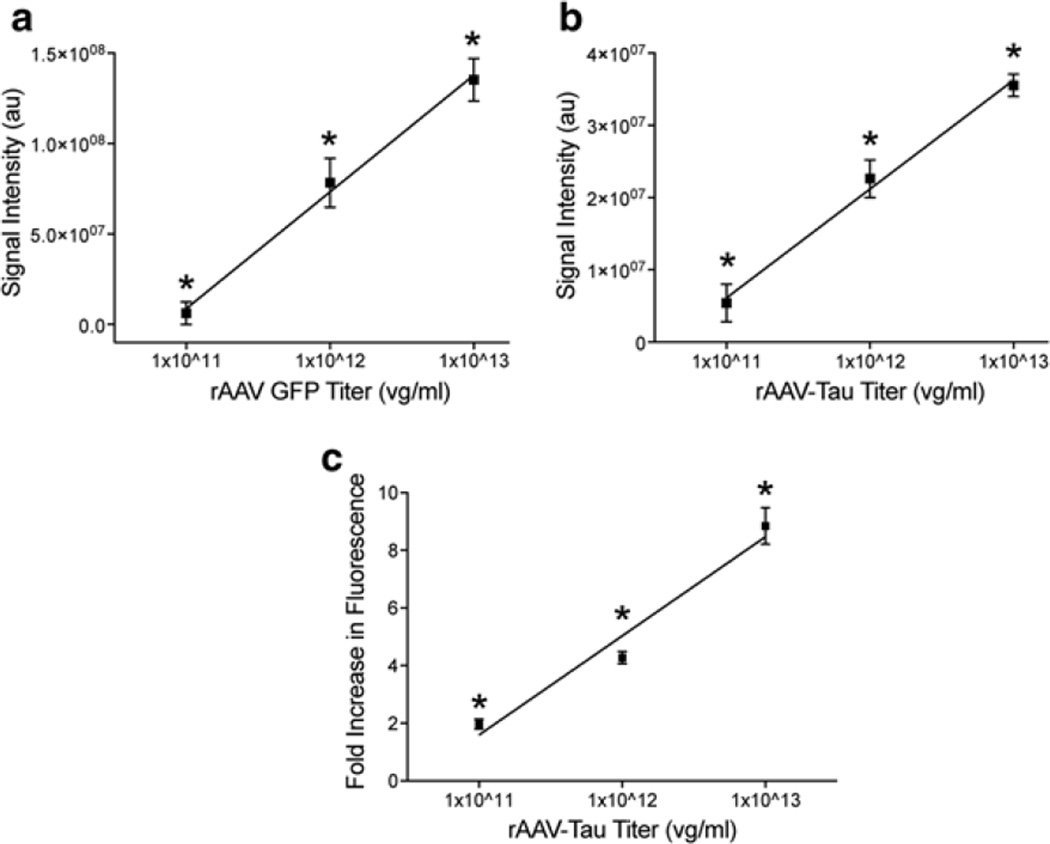
Every model system has some limitations that are important to acknowledge. rAAVs efficiently transduce cells in the brain, but the level of overexpression can be significant. However, two approaches can help minimize this effect. First, rAAVs can be titered to effectively reduce the copy number per cell, thereby reducing the amount of expression. For example, injection of 2 µl of rAAV-GFP or rAAV-human wild-type tau at a titer of 1 × 1013, 1 × 1012 or 1 × 1011 viral genomes/ml into the dorsal HP of rats (as above) results in a dose-response of expression levels. The regional HP signal for GFP or human tau levels (using Licor Odyssey densitometry analysis) decreased linearly (GFP - r2 = 0.92, p < 0.001; human tau - r2 = 0.93 p < 0.001; Fig. 3a, b). Analysis of tau fluorescence levels in individual cells found that the average level of tau expression was approximately ninefold, fourfold or twofold over endogenous tau levels at titers of 1 × 1013, 1 × 1012 or 1 × 1011, respectively (Fig. 3c). Second, regulatable promoter systems, such as tetracycline induction systems, provide an effective level of control over transgene expression [85] but leakiness is always a concern with these approaches (as with regulatable transgenic animals). Nonetheless, it is imperative that investigators assess the level of overexpression when using any model system.
Fig. 3.
rAAV2/5 titer-dependent changes in levels of transgene expression and fold-increase in expression are linear in the rat hippocampus (HP). (a) Young Fischer 344 rats (3 months, n = 3/group) were injected in the dorsal HP with rAAV-GFP at 1 × 1011, 1 × 1012, or 1 × 1013 vg/ml (as described in Fig. 1). After 1 month of expression, the tissue was processed for GFP immunofluorescence using GFP antibody (1:28,000) and Licor goat anti-rabbit IRDye 680 (1:500). The fluorescence signal intensity was quantified using Licor Odyssey densitometry analysis in the dorsal hippocampus. Increasing the titer produced a linear increase (r2 = 0.92, p < 0.001) in regional GFP signal intensity. Each increase in titer produces a significant increase in signal (one-way ANOVA, *p < 0.05 vs. other groups). (b) Using the same parameters, animals (n = 3/group) were injected with rAAV-human tau at the three titer doses. Again, a linear increase (r2 = 0.93, p < 0.001) in regional human tau (Tau12; 1:40,000, Licor goat anti-mouse 680) signal intensity was observed with increasing titers (using Licor densitometry). Each increase in titer produces a significant increase in signal (one-way ANOVA, *p < 0.05 vs. other groups). (c) Sections from rAAV-human tau animals were stained with dual immunofluorescence for Tau5 (a mouse monoclonal pan-tau antibody that labels human and rodent tau equally, 1:50,000; [147]) and a rabbit anti-flag tag antibody (Sigma, F7425, 1:2,000) to obtain estimations of the level of tau overexpression on an individual cell basis. The human tau proteins are flag tagged, which is a small epitope tag (DYKDDDDK), to facilitate distinction of the human tau from endogenous rat tau. Transduced cells were identified with the flag tag staining and levels of Tau5 fluorescence were measured in the cytoplasm of individual neurons. Normal physiological levels of endogenous rat tau were measured in non-transduced cells (i.e., flag tag negative) in the contralateral hemisphere. The data are presented as the fold-increase in fluorescence intensity (i.e., tau protein levels) from non-transduced cells. As with the regional analyses, a linear increase in fluorescence was observed with increasing titers (r2 = 0.93, p < 0.001). The range of the fold-increase in expression within individual cells was 1–3 for 1 × 1011, 2–14 for 1 × 1012, and 2–17 for 1 × 1013. Each increase in titer produces a significant increase in signal (one-way ANOVA, *p < 0.05 vs. other groups). Of note, this method can only approximate relative expression levels and does not allow precise quantitative measures of protein levels in each cell. Also, methods that do not allow single-cell analysis (e.g., western blotting or ELISAs) are inappropriate for estimating overexpression levels as the samples contain significant quantities of non-transduced cells/tissue. Finally, the reduction in viral titer reduces expression levels, but also decreases the amount of transduced neurons. Thus, one must balance the level of overexpression with transducing an adequate number of cells
Another potential caveat of rAAVs is the lack of absolute specificity in cell-type transduction. Preferential tropism of certain cell types does occur with different rAAV serotypes, but some tropism for other cell types exists as well. For example, rAAV 2/5 is typically described as a neuron-specific serotype, however, a low level of glial transduction is often observed. One mechanism to overcome this is to utilize cell-type specific promoters. Viruses that utilize neuron-specific (e.g., synapsin I) or glial-specific (e.g., glial fibrillary acidic protein) promoters can help address this caveat [87, 93]. Moreover, cell phenotype-specific markers (e.g., tyrosine hydroxylase promoter for monoaminergic neurons) can increase specificity to neuronal subpopulations. As the use of rAAVs becomes more common in the AD and tauopathy field, it will be important to pursue these more refined approaches.
Finally, delivery of rAAV into the brain requires surgical interventions, which lead to disruption of the blood–brain barrier and the brain tissues. To reduce disruption, it is advisable to perform the stereotaxic surgery with the aid of a surgical scope and pulled glass syringe tips. Often, this allows minimal disruption of the dura and in some cases the only disruption is the very fine point of the inserting syringe tip. Details on the surgical procedures used for delivering rAAVs intracerebrally can be found in Chapter 14. Additionally, recent evidence suggests that rAAV 2/9 can effectively enter the brain after a peripheral injection, thereby, circumventing the need for surgery [94].
6 AD and Other Tauopathy Models Using rAAV Tau
The initial rAAV tauopathy models used rAAV-2/2 to express full-length human tau with the P301L mutation in the brains of mice and rats [43, 95]. rAAV-P301L tau was injected into the basal forebrain of adult rats leading to detection of increased levels of the tau protein in the brain for at least 8 months after injection. Within 3–4 weeks hyperphosphorylated tau was found and aggregates resembling NFTs were present although in relatively low numbers. These studies provided the first proof-of-principle that injection of rAAV-P301L tau could result in persistent expression of tau protein and tau aggregation in rodents.
Injection of rAAV-wild-type or triple-mutant APP led to some Aβ plaques but no overt neurodegeneration. In contrast, rAAV-wild-type tau or P301L tau caused significant neurodegeneration of HP pyramidal neurons, but tangles were not present [96]. The tau-induced neurodegeneration was dependent on the microtubule-binding domains, as a truncated version of the protein did not induce toxicity. Interestingly, P301L tau apparently induced cell cycle reentry as indicated by an increase in the presence of cell cycle markers (e.g., cyclins B1 and D2, proliferating cell nuclear antigen, phosphorylated retinoblastoma protein). However, the neurodegenerative effects of injecting rAAV-wild-type tau into the dorsal HP were not replicated in another study using rats [97]. Differences in species, age at rAAV delivery, duration of expression, viral titer and/or neuroinflammatory response may help to explain the discordant findings between these studies.
In a recent experiment, our group used rAAV2/5 to express GFP (used as a control rAAV) or wild-type human tau (longest 4R isoform of 441 amino acids) in the dorsal HP of old Fischer 344 rats (20 months) for 1 month (Fig. 4). Both constructs were well expressed by HP neurons (CA1 pyramidal neurons depicted). Notably, there were clear signs of axonal degeneration, such as fragmentation of tau expressing HP axons in the fimbria, while GFP expressing axons appeared normal. Moreover, the pattern of HP staining with SMI312 antibody, an axon-specific neurofilament antibody cocktail, showed an apparent reduction in axons and the remaining axons appeared dystrophic and fragmented. These data support the usefulness of using rAAV approaches to model early pathological changes such as axonal degeneration and highlight the ability to use aged rats in such studies.
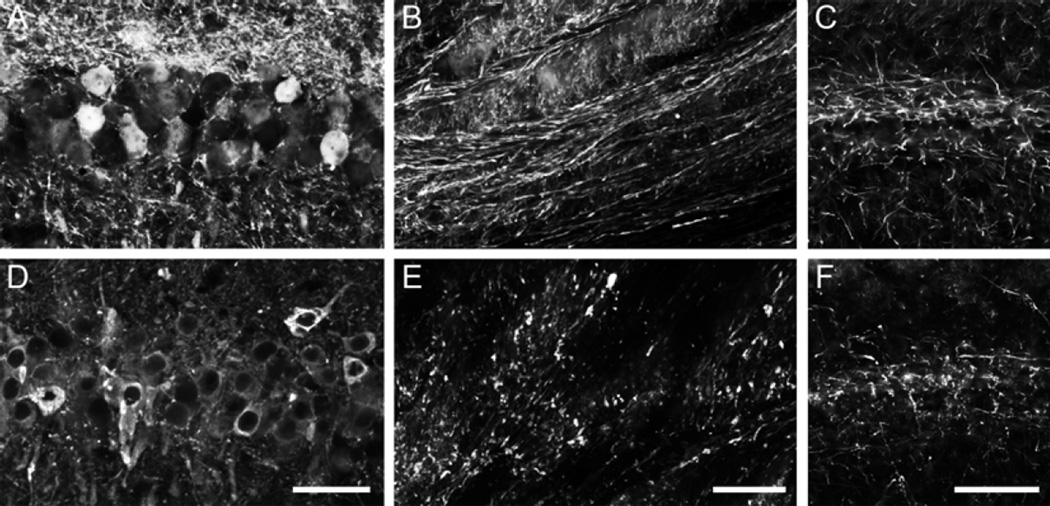
Fig. 4.
Expression of human tau using rAAV2/5 causes axonal degeneration in the aged rat hippocampus (HP). Aged Fischer 344 rats (20 months, n = 3/group) were injected in the dorsal HP (as described in Fig. 1) with either rAAV-GFP (a–c) or rAAV-human tau (d–f). (a) and (d) The CA1 pyramidal neurons were well transduced in both cases (a —GFP fluorescence and d —Tau12 immunofluorescence). (b) and (e) Examination of the HP-derived axons in the fimbria revealed that GFP+ axons appeared normal and continuous (b), while human tau + axons showed clear signs of degeneration (e.g., fragmentation and dystrophy). (c) and (f) SMI312, an axon-specific neurofilament monoclonal antibody cocktail (Covance, SMI-312R, 1:10,000) revealed an apparent reduction and fragmentation of axons in the HP (CA1 region depicted) of aged animals expressing human tau (f) when compared to those expressing GFP (e). Scale bars in d, e, and f = 50 µm. Of note, overt neuron loss in the HP was not assessed in this study
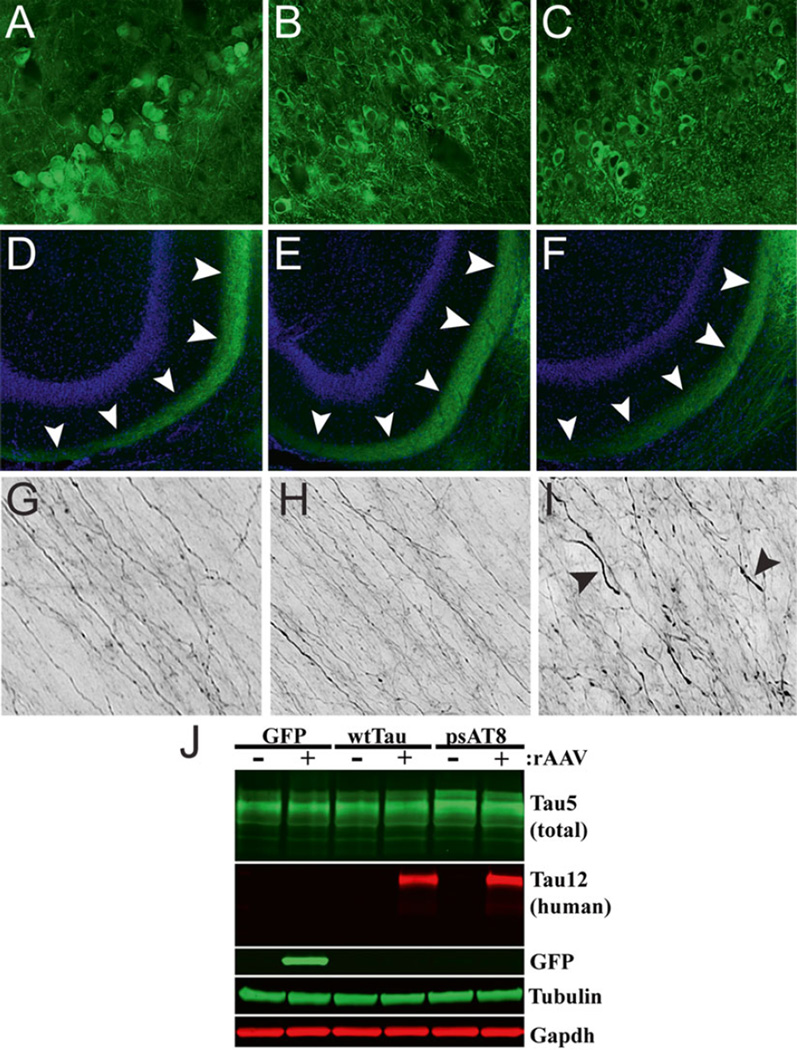
The perforant pathway projects from the EC to the HP and is associated with memory formation [98]. It is also the location of some of the earliest tau pathology in AD and among the regions that undergo significant neurodegeneration [99]. rAAV vectors provide an opportunity to model the early stages of AD and the degeneration associated with the perforant pathway [100]. Expression of rAAV2/9-P301L under the control of a synapsin I promoter produced localized tau expression in the lateral EC and perforant pathway. Multiple forms of phosphorylated and aggregated tau were detected in this region by antibodies and silver staining prior to loss of perforant synapses and neuronal death, seemingly triggered through caspase-mediated apoptosis. HP tau expression was initially present only in the perforant pathway projections but was later observed in the dentate granule neurons as well as in target neurons in CA3. This progressive expression was not replicated in rAAV-eGFP controls and may support trans-synaptic spread of tau [100]. We had similar success in efficiently transducing EC neurons in young (3–4 months) Fischer 344 rats using rAAV-2/5. In this experiment, rAAV-wild-type human tau and a phosphomimetic form of AT8 tau (i.e., pseudophosphorylations at S199, S202, and T205, rAAV-psAT8) was injected into the EC of young animals. AT8 tau is a prominent disease-related phosphoepitope of tau and recombinant psAT8 tau significantly impaired anterograde axonal transport as a monomer in the squid axoplasm, an effect not seen with wild-type tau monomers in this assay [19]. Notably, expression of GFP or wild-type tau for 1 month did not appear to alter perforant pathway axons in young animals (Fig. 5a), but AT8 tau expression induced early signs of axonal degeneration (e.g., spheroids and dystrophic axons) in perforant pathway axons (Fig. 5b). One notable caveat of directly injecting the rodent EC is that the syringe tip must pass through the HP (at an angle). Thus, this approach is not well-suited for studying cell-to-cell transfer or the spread of tau because of the likelihood for injected materials to flow back along the needle tract. Nonetheless, the EC can be targeted and overexpression of disease-related forms of tau (i.e., psAT8) appear to induce axonopathy in young animals. Further investigations that study the long-term and aging-related effects of rAAV tau expression in the EC should be pursued.
Fig. 5.
Expression of a disease-related phosphomimetic form of human tau in the entorhinal cortex (EC) of young rats causes axonal abnormalities in the perforant pathway. Young Fischer 344 rats were injected with rAAV-GFP (a, d, g), rAAV-human tau (b, e, h), or rAAV human tau pseudophosphorylated at the AT8 site (psAT8—S199, S202 and T205) into the EC (c, f, i; as described in Fig. 2; titer = 1.9 × 1013 for all). (a–f) After 1 month, pyramidal neurons of the EC (a–c) were transduced and proteins were present in the outer molecular layer of the dentate gyrus (arrowheads in d–f), which is a terminal field of the axonal projections from the EC. (g–i) Notably, axons within the perforant pathway appeared normal in GFP and human tau expressing animals, but in psAT8 tau expressing animals axons appeared dystrophic (arrowheads; i.e., swollen and containing spheroids). Endogenous GFP fluorescence (a, d, g) or Tau12 immunofluorescence (b, c, e, f, h, i) were used to identify rAAV-GFP or rAAV-human tau and rAAV-psAT8, respectively. (j) Western blot was used to confirm the expression of rAAV-GFP, rAAV-human tau and rAAV-AT8 tau in the EC (+ samples are the injected side, - samples are the contralateral HP). Immunoblotting antibodies were used as described in Fig. 1, and Tau5 was used at 1:100,000. Overt neurodegeneration was not assessed in this study
7 Tau in the Substantia Nigra
The presence of tau pathology and neuronal loss is observed in the SN in many tauopathies, including PSP, CBD, FTDP-17, as well as AD (around Braak Stage VI) and Parkinson’s disease [101–104]. rAAV2/2-mediated expression of wild-type or P301L tau in the SN of rat caused early axonal degeneration that was followed by neuronal loss [105]. Accordingly, the animals displayed motor deficits and a directional bias in amphetamine-induced rotational behavior. These behavioral changes occurred in the absence of large numbers of NFT-like inclusions, indicating that tangle-like inclusions may not be required for neuronal dysfunction. In another study, a number of different rAAV serotypes (i.e., rAAV2/2, rAAV2/5, rAAV2/8, rAAV2/9, and rAAV2/10) were tested with P301L tau in the SN [106, 107]. rAAV2/8 P301L induced high tau expression and a loss of approximately 75 % of TH-positive neurons in the SN, while rAAV2/2 and rAAV2/5 induced losses of less than 30 % [106]. rAAV2/9 and rAAV2/10 induced high tau expression and led to the loss of around 90 % of dopaminergic neurons [107]. Interestingly, GFP expression through rAAV2/8 transduction was toxic in the SN at high doses but not in the HP or when expressed through rAAV2/9 [106, 107]. These studies highlight the importance of choosing appropriate control proteins and potential issues with nonspecific toxicity due to dosing effects. Given the losses of 75–90 % of dopaminergic neurons in the SN, this may be of use as a model for some tauopathies that display that particular phenotype, such as PSP, CBD, or FTDP-17 [37, 108, 109].
A study found rAAV2/9 wild-type tau or GFP (at titers of either 2 × 109 or 9 × 109) produced similar levels of expression in young and aged rats in the SN [83, 84]. rAAV-tau expression in the SN caused prolonged microglial activation, neuronal loss and amphetamine-induced rotational behavior in the young and old animals. Interestingly, this effect was enhanced in older rats at lower titers suggesting aged animals are more susceptible to tau toxicity. In fact, expression of wild-type tau with rAAV2/9 induced an upregulation of pro-inflammatory markers and reduced tyrosine hydroxylase mRNA due to loss of dopamine neurons [110], suggesting neuroinflammation might play an important role in tau toxicity in the SN.
8 Mixed Models Using rAAV Tau and Transgenic Mice
In addition to developing novel models, viral transduction can help to improve on existing transgenic models by expressing proteins that introduce or enhance pathology. For example, the PS/APP double transgenic mouse develops amyloid pathology, but only low levels of tau pathology [63]. However, injection of rAAV-P301L tau into the HP of these mice produced moderate tau pathology including early stage tau aggregates and tau-positive neurites surrounding amyloid deposits [95]. Similarly, injection of rAAV2/2-P301L tau into the HP of the triple-mutant APP mouse line, mThy1-hAPP751, resulted in increased phosphorylated tau and tau fibrillar pathology compared to wild-type mice injected with rAAV-P301L tau [58, 111]. Moreover, rAAV-P301L tau induced neuronal loss in the transgenic mice that was not seen in either the wild-type mice or transgenic mice injected with rAAV-GFP, an effect that was attenuated through treatment with a kinase inhibitor [111]. These studies highlight the benefits of combining transgenic mice with rAAV injections to study disease mechanisms and potential therapeutics.
In another series of studies, the TASTPM transgenic mouse line (an APP/PS1 mouse) [112, 113] was injected in the EC with rAAV6 containing wild-type tau, P301S mutant tau, or a modified, enhanced aggregating version of tau named 3PO [114, 115]. The pro-aggregation forms of tau, P301S and 3PO tau, enhanced toxicity around the injection site as well as in CA1 HP neurons and exacerbated microglial activation when compared to wild-type tau. Importantly, the expression of rAAV P301S or 3PO tau resulted in impaired spatial memory. This confirmed previous results demonstrating that transduction of mutant tau into the EC impaired spatial memory of mice [116].
Combining different transgenic lines with rAAV approaches can add novel capability to studying specific processes related to a disease. For example, yellow fluorescent protein transgenic mice were injected with rAAV-P301L tau into the HP, providing a model for tracking axonal degeneration and neuronal loss [117]. In the same study, rAAV-P301L was injected into the HP of transgenic mice harboring the CX3C chemokine receptor 1 gene disrupted with EGFP (produces mice with GFP+ microglia). This study confirmed early degeneration of neuronal processes (i.e., axons and dendrites) prior to overt neuronal degeneration and activation of microglia was detected after the induction of tau expression. These results supported a toxic role for tau prior to the formation of NFT-like aggregates as well as a neurotoxic effect related to microglia.
9 rAAV-Mediated Expression of Other AD- and Dementia-Related Proteins
A number of groups have used rAAVs to model other pathological aspects of AD. For example, rAAVs were used to overexpress Aβ peptides in rodents [118]. Expression of rAAV2/1-BRI-Aβ42, a fusion protein that allows controlled expression and secretion of Aβ peptides [119, 120], in the HP induced the formation of plaques but this was not seen with rAAV2/1-BRI-Aβ40 expression. Another amyloid-based rAAV study generated GFP-tagged versions of Aβ40 and Aβ42, wild-type C100 (a C-terminal fragment of APP containing the Aβ peptides) and a V717F mutant version of C100 and expressed them in the HP and cerebellum of mice using rAAV2/2 [121]. The Aβ42 and V717F C100 led to greater induction of microgliosis and disruption of the blood–brain barrier compared to the Aβ40 forms but did not induce plaque formation, neurodegeneration or astrocyte activation. The use of rAAV technology to express various forms of Aβ and its precursor proteins will continue to be an important method of studying their role in disease.
Similarly, rAAVs were used to induce expression or alteration of other proteins thought to play a role in the mechanisms of AD. Injection of rAAV1-I2NTF and rAAV1-I2CTF, active cleavage products of an inhibitor of protein phosphatase-2A (PP2A), led to phosphorylation and aggregation of tau and Aβ, synaptic defects, and impaired memory in rats [122]. This is an intriguing demonstration of induction of some of the pathological hallmarks of AD without overexpressing mutant forms of tau or APP. Another novel method used rAAV1/2 to express 5-Lipoxygenase (5-LO) in the brains of transgenic APP (Tg2576) or 3×Tg-AD mice resulting in impaired performance in memory tasks and exacerbated Aβ and tau pathology [123, 124]. 5-LO in an enzyme that oxidizes lipids, such as arachidonic acid, releasing several active compounds into the cell and displays increased levels in the HP of AD patients [125].
Some FTDs are characterized by pathological aggregation of TDP-43, a nucleic acid-binding protein, primarily in the frontotemporal cortex and HP (reviewed in Ref. [126]). Overexpression of rAAV2/9-TDP-43 in the SN of rats caused amphetamine-induced asymmetrical rotational behavior, loss of TH-positive neurons, and the development of cytoplasmic TDP-43 aggregates [127]. Similarly, rAAV2/9-TDP-43 expression in the rat HP caused neuronal loss and some memory impairments [97]. Another study took advantage of the flexible nature of rAAVs to address differential toxicities and pathologies due to expression of mutant TDP-43 in neurons and glia of mice [128]. The synapsin 1 and GFAP promoters limited expression of the M337V TDP-43 to neurons and astrocytes, respectively. Neuronal expression resulted in greater TDP-43 aggregation, neuronal loss, and more severe motor defects in the mice. Astrocytic expression also induced neuronal death but only in older animals and without motor defects. These studies demonstrate that rAAV models are a versatile approach to studying the role of pathological proteins as well as the molecular mechanisms of disease, and may help make important distinctions between different cell-specific pathologies.
10 Lentiviral Models
Other models of AD and related neurodegenerative disorders utilize lentiviral vectors, a retrovirus, to express the desired protein as an alternative to rAAV. These differ from rAAVs because they insert themselves into the host genome and have a capacity for larger genetic material. Some of the AD and tauopathy models developed using lentiviral systems are discussed briefly below.
A number of studies used lentiviruses to express wild-type or mutant forms of tau protein in various brain regions of rodents. Lentivirus -mediated expression of P301S tau in the HP of wild-type or transgenic APP23 mice (harboring 2 Swedish APP mutations) was used to examine interactions between tau and Aβ in adult mice [129]. Based on immunostaining, the APP23 transgenic mice displayed increased tau phosphorylation and aggregation compared to the wild-type mice also injected with lenti-P301S. Another group found that P301L tau expression caused an increase in tau phosphorylation, tau aggregation, and activated microglia when compared to wild-type tau expression in the rat motor cortex rats [130]. Lentivirus expression of wild-type or P301L tau in the rat HP produced a progressive appearance of dystrophic and fragmented neurites prior to the formation of NFT-like inclusions that developed over 8 months [131]. Lentiviral expression of wild-type tau, P301L, and Aβ42 led to microglial activation as well as induction of autophagy as fractalkine signaling was increased [132]. This indicates a possible link between the phosphorylated tau, microglia signaling, and induction of autophagy that could be related to apoptotic death of neurons. Finally, lentiviral Aβ42 led to intracellular Aβ accumulation followed by tau hyperphosphorylation that was ameliorated with clearance of the peptide [133].
Lentiviruses were used to express TDP-43, Aβ42, or both together in the motor cortex of rats [134]. Lenti-TDP-43 induced caspase activity, neuroinflammation, and altered the normal processing of APP, which led to increased Aβ42 production, while lenti-Aβ42 induced some caspase-3 activity and neuroinflammation. Interestingly, the combined expression of TDP-43 and Aβ42 produced pathology similar to TDP-43 alone but overt neuronal loss occurred, indicating a possible synergistic effect between the two proteins. Another study using lentiviruses to express TDP-43 in the motor cortex found altered amino acid metabolism, oxidative stress, and neuronal death [135].
The studies described above demonstrate the versatility and utility of using rAAV and lentiviruses to produce models of neurodegenerative diseases. These tools can be used alone or in conjunction with existing transgenic models to help address potential pitfalls of previous modeling techniques. These valuable techniques will continue to provide insight into neurodegenerative diseases by allowing elucidation of mechanisms and providing platforms to explore new therapeutics.
11 Possible Therapeutic Uses of Viral Vectors
Currently, there are no treatments that effectively slow or prevent AD and other tauopathies. While the precise mechanisms of degeneration are unknown, loss of cholinergic neurons in the basal fore-brain and excitotoxicity are believed to contribute to the development of AD [136, 137]. Accordingly, cholinesterase inhibitors (donepezil, rivastigmine, tacrine, and galantamine), which reduce acetylcholine breakdown, are used to treat mild to moderate AD. In addition, a noncompetitive NMDA receptor antagonist (memantine) is prescribed for moderate to severe disease stages [138]. However, neither of these drugs slow or prevent disease progression and only provide temporary symptomatic improvement for some patients [139]. Thus, there is an urgent need to aggressively develop novel therapeutics for treating AD and other tauopathies.
Nerve growth factor (NGF) is an appealing treatment because it potently protects cholinergic neurons and improves the function of basal forebrain neurons [139, 140]. The delivery of NGF to the nucleus basalis of Meynert was approached by direct infusion into the brain, implantation of cell grafts of NGF-secreting fibroblasts, and viral-vector delivery using adeno-associated virus. NGF injection into the lateral cerebral ventricle led to pain syndrome and weight loss with no obvious cognitive improvement in patients [141]. The delivery of NGF via fibroblast provided some therapeutic benefits in nonhuman primates, but the complexity of manufacturing fibroblasts and the diminished protein expression after 18 months makes it less desirable [142]. Using a rAAV vector to deliver NGF to the target region of the forebrain eliminates the hurdles of other NGF delivery methods. A phase I clinical trial (NCT00087789) was conducted using CERE-110 for the treatment of AD patients. The CERE-110 viral vector is an AAV2/2 vector containing full length NGF transgene under the control of the CMV/β-actin promoter and the human polyadenylation signal [143]. There were few adverse effects of the treatment reported, and all were deemed either not related or unlikely related to the rAAV-NGF treatment [139]. The phase I trial confirmed that viral vector therapy was well tolerated with a high level of safety and no systemic toxicity. The success of the phase I trial led to a phase II clinical trial using CERE-110 and results are expected in 2015. As our knowledge of disease mechanisms continues to grow and novel therapies are developed, gene therapy must continue to be pursued as a viable approach for therapeutic interventions in AD and other tauopathies.
Acknowledgments
This work was supported by NIH/NIA grant R01AG044372 (N.M.K.).
References
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease [see comments] Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 4.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F, Sisodia SS, Greengard P, Xu H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc Natl Acad Sci U S A. 1999;96(2):742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156(1):15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 8.Duering M, Grimm MO, Grimm HS, Schroder J, Hartmann T. Mean age of onset in familial Alzheimer’s disease is determined by amyloid beta 42. Neurobiol Aging. 2005;26(6):785–788. doi: 10.1016/j.neurobiolaging.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26(22):6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27(11):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin N, Romero B, Bosch-Morell F, Llansola M, Felipo V, Roma J, Romero FJ. Beta-amyloid-induced activation of caspase-3 in primary cultures of rat neurons. Mech Ageing Dev. 2000;119(1–2):63–67. doi: 10.1016/s0047-6374(00)00172-x. [DOI] [PubMed] [Google Scholar]
- 12.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418(6895):291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 13.Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intra-neuronal amyloid beta. Proc Natl Acad Sci U S A. 2009;106(14):5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta-amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A. 2002;99(9):6364–6369. doi: 10.1073/pnas.092136199. 092136199 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. 316/5825/750 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. S0092-8674(10)00726-9 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360(6405):674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- 19.Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, Binder LI. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011;31(27):9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111(Pt 21):3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 21.Kosik KS, Crandall JE, Mufson EJ, Neve RL. Tau in situ hybridization in normal and Alzheimer brain: localization in the somato-dendritic compartment. Ann Neurol. 1989;26(3):352–361. doi: 10.1002/ana.410260308. [DOI] [PubMed] [Google Scholar]
- 22.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261(13):6084–6089. [PubMed] [Google Scholar]
- 24.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 25.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Heutink P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 26.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 27.Kowall NW, Kosik KS. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer’s disease. Ann Neurol. 1987;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- 28.Bondareff W, Mountjoy CQ, Roth M, Hauser DL. Neurofibrillary degeneration and neuronal loss in Alzheimer’s disease. Neurobiol Aging. 1989;10(6):709–715. doi: 10.1016/0197-4580(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 29.Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol. 1999;58(2):188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML, Spires-Jones TL, Bacskai BJ, Hyman BT. Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci U S A. 2014;111(1):510–514. doi: 10.1073/pnas.1318807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muoz MJ, Jackson GR, Kayed R. Preparation and characterization of neurotoxic tau oligomers. Biochemistry. 2010;49(47):10039–10041. doi: 10.1021/bi1016233. [DOI] [PubMed] [Google Scholar]
- 32.Tian H, Davidowitz E, Lopez P, Emadi S, Moe J, Sierks M. Trimeric tau is toxic to human neuronal cells at low nanomolar concentrations. Int J Cell Biol. 2013;2013:260787. doi: 10.1155/2013/260787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flach K, Hilbrich I, Schiffmann A, Gartner U, Kruger M, Leonhardt M, Waschipky H, Wick L, Arendt T, Holzer M. Tau oligomers impair artificial membrane integrity and cellular viability. J Biol Chem. 2012;287(52):43223–43233. doi: 10.1074/jbc.M112.396176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vana L, Kanaan NM, Ugwu IC, Wuu J, Mufson EJ, Binder LI. Progression of tau pathology in cholinergic Basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. Am J Pathol. 2011;179(5):2533–2550. doi: 10.1016/j.ajpath.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee VM, Trojanowski JQ. Neurodegenerative tauopathies: human disease and transgenic mouse models. Neuron. 1999;24(3):507–510. doi: 10.1016/s0896-6273(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 37.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44(11):2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 38.Cervos-Navarro J, Schumacher K. Neurofibrillary pathology in progressive supranuclear palsy (PSP) J Neural Transm Suppl. 1994;42:153–164. doi: 10.1007/978-3-7091-6641-3_12. [DOI] [PubMed] [Google Scholar]
- 39.Munoz-Garcia D, Ludwin SK. Classic and generalized variants of Pick’s disease: a clinicopathological, ultrastructural, and immunocytochemical comparative study. Ann Neurol. 1984;16(4):467–480. doi: 10.1002/ana.410160408. [DOI] [PubMed] [Google Scholar]
- 40.Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18(1):20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- 41.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee HS, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2012;136(Pt 1):43–64. doi: 10.1093/brain/aws307. aws307 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Combs B, Gamblin TC. FTDP-17 tau mutations induce distinct effects on aggregation and microtubule interactions. Biochemistry. 2012;51:8597–8607. doi: 10.1021/bi3010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Gong CX. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener. 2008;3:8. doi: 10.1186/1750-1326-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci Biobehav Rev. 2011;35(6):1397–1409. doi: 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bamburg JR, Bloom GS. Cytoskeletal pathologies of Alzheimer disease. Cell Motil Cytoskeleton. 2009;66(8):635–649. doi: 10.1002/cm.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolan PJ, Johnson GV. The role of tau kinases in Alzheimer’s disease. Curr Opin Drug Discov Devel. 2010;13(5):595–603. [PMC free article] [PubMed] [Google Scholar]
- 49.Takalo M, Salminen A, Soininen H, Hiltunen M, Haapasalo A. Protein aggregation and degradation mechanisms in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 50.Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5(3):421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanaan NM, Pigino GF, Brady ST, Lazarov O, Binder LI, Morfini GA. Axonal degeneration in Alzheimer’s disease: when signaling abnormalities meet the axonal transport system. Exp Neurol. 2013;246:44–53. doi: 10.1016/j.expneurol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armstrong RA. What causes Alzheimer’s disease? Folia Neuropathol. 2013;51(3):169–188. doi: 10.5114/fn.2013.37702. [DOI] [PubMed] [Google Scholar]
- 54.Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9(7):532–544. doi: 10.1038/nrn2420. nrn2420 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Elder GA, Gama Sosa MA, De Gasperi R. Transgenic mouse models of Alzheimer’s disease. Mt Sinai J Med. 2010;77(1):69–81. doi: 10.1002/msj.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(11):a006320. doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276(24):21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 58.Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1-42) J Neurosci Res. 2001;66(4):573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 59.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 60.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 61.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94(24):13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 63.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 64.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13(2):159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 65.Siman R, Reaume AG, Savage MJ, Trusko S, Lin YG, Scott RW, Flood DG. Presenilin-1 P264L knock-in mutation: differential effects on abeta production, amyloid deposition, and neuronal vulnerability. J Neurosci. 2000;20(23):8717–8726. doi: 10.1523/JNEUROSCI.20-23-08717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 68.Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci. 2013;33(15):6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price DL, Tang F, Markowska AL, Borchelt DR. Episodic-like memory deficits in the APPswe/ PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18(3):602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 70.McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, Leverenz J, Schellenberg GD, D’Souza I. Tau isoform regulation is region- and cell-specific in mouse brain. J Comp Neurol. 2008;511(6):788–803. doi: 10.1002/cne.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanes J, Zilka N, Bartkova M, Caletkova M, Dobrota D, Novak M. Rat tau proteome consists of six tau isoforms: implication for animal models of human tauopathies. J Neurochem. 2009;108(5):1167–1176. doi: 10.1111/j.1471-4159.2009.05869.x. [DOI] [PubMed] [Google Scholar]
- 72.Goedert M, Crowther RA, Spillantini MG. Tau mutations cause frontotemporal dementias. Neuron. 1998;21(5):955–958. doi: 10.1016/s0896-6273(00)80615-7. [DOI] [PubMed] [Google Scholar]
- 73.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. S0896-6273(07)00030-X [pii] [DOI] [PubMed] [Google Scholar]
- 74.Tanemura K, Akagi T, Murayama M, Kikuchi N, Murayama O, Hashikawa T, Yoshiike Y, Park JM, Matsuda K, Nakao S, Sun X, Sato S, Yamaguchi H, Takashima A. Formation of filamentous tau aggregations in transgenic mice expressing V337M human tau. Neurobiol Dis. 2001;8(6):1036–1045. doi: 10.1006/nbdi.2001.0439. S0969-9961(01)90439-5 [pii] [DOI] [PubMed] [Google Scholar]
- 75.Gotz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, Goedert M. Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J. 1995;14(7):1304–1313. doi: 10.1002/j.1460-2075.1995.tb07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25(22):5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. 25/22/5446 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73(4):685–697. doi: 10.1016/j.neuron.2011.11.033. S0896-6273(12)00038-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7(2):e31302. doi: 10.1371/journal.pone.0031302. PONE-D-11-23353 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein RL, Wang DB, King MA. Versatile somatic gene transfer for modeling neurodegenerative diseases. Neurotox Res. 2009;16(3):329–342. doi: 10.1007/s12640-009-9080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Low K, Aebischer P. Use of viral vectors to create animal models for Parkinson’s disease. Neurobiol Dis. 2012;48(2):189–201. doi: 10.1016/j.nbd.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 81.Terzi D, Zachariou V. Adeno-associated virus-mediated gene delivery approaches for the treatment of CNS disorders. Biotechnol J. 2008;3(12):1555–1563. doi: 10.1002/biot.200800284. [DOI] [PubMed] [Google Scholar]
- 82.Polinski NK, Gombash SE, Manfredsson FP, Lipton JW, Kemp CJ, Cole-Strauss A, Kanaan NM, Steece-Collier K, Kuhn NC, Wohlgenant SL, Sortwell CE. Recombinant adenoassociated virus 2/5-mediated gene transfer is reduced in the aged rat midbrain. Neurobiol Aging. 2014;36:1110–1120. doi: 10.1016/j.neurobiolaging.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klein RL, Dayton RD, Diaczynsky CG, Wang DB. Pronounced microgliosis and neurodegeneration in aged rats after tau gene transfer. Neurobiol Aging. 2010;31(12):2091–2102. doi: 10.1016/j.neurobiolaging.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu K, Meyers CA, Guerra NK, King MA, Meyer EM. The effects of rAAV2-mediated NGF gene delivery in adult and aged rats. Mol Ther. 2004;9(2):262–269. doi: 10.1016/j.ymthe.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 85.Manfredsson FP, Burger C, Rising AC, Zuobi-Hasona K, Sullivan LF, Lewin AS, Huang J, Piercefield E, Muzyczka N, Mandel RJ. Tight Long-term dynamic doxycycline responsive nigrostriatal GDNF using a single rAAV vector. Mol Ther. 2009;17(11):1857–1867. doi: 10.1038/mt.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J, Ehlers MD, Zylka MJ, McCown TJ, Samulski RJ. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther. 2011;22(9):1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.von Jonquieres G, Mersmann N, Klugmann CB, Harasta AE, Lutz B, Teahan O, Housley GD, Frohlich D, Kramer-Albers EM, Klugmann M. Glial promoter selectivity following AAV-delivery to the immature brain. PLoS One. 2013;8(6):e65646. doi: 10.1371/journal.pone.0065646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22(21):9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8(9):e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 91.Gao G, Sena-Esteves M. Introducing genes into mammalian cells: viral vectors. In: Green MR, Sambrook J, editors. Molecular cloning: a laboratory manual, vol 2, 4. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2012. pp. 1209–1333. [DOI] [PubMed] [Google Scholar]
- 92.Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003;100(4):1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kugler S, Kilic E, Bahr M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 2003;10(4):337–347. doi: 10.1038/sj.gt.3301905. [DOI] [PubMed] [Google Scholar]
- 94.Rahim AA, Wong AM, Hoefer K, Buckley SM, Mattar CN, Cheng SH, Chan JK, Cooper JD, Waddington SN. Intravenous administration of AAV2/9 to the fetal and neonatal mouse leads to differential targeting of CNS cell types and extensive transduction of the nervous system. FASEB J. 2011;25(10):3505–3518. doi: 10.1096/fj.11-182311. [DOI] [PubMed] [Google Scholar]
- 95.Klein RL, Lin WL, Dickson DW, Lewis J, Hutton M, Duff K, Meyer EM, King MA. Rapid neurofibrillary tangle formation after localized gene transfer of mutated tau. Am J Pathol. 2004;164(1):347–353. doi: 10.1016/S0002-9440(10)63124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jaworski T, Dewachter I, Lechat B, Croes S, Termont A, Demedts D, Borghgraef P, Devijver H, Filipkowski RK, Kaczmarek L, Kugler S, Van Leuven F. AAV-tau mediates pyramidal neurodegeneration by cell-cycle re-entry without neurofibrillary tangle formation in wild-type mice. PLoS One. 2009;4(10):e7280. doi: 10.1371/journal.pone.0007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dayton RD, Wang DB, Cain CD, Schrott LM, Ramirez JJ, King MA, Klein RL. Frontotemporal lobar degeneration-related proteins induce only subtle memory-related deficits when bilaterally overexpressed in the dorsal hippocampus. Exp Neurol. 2012;233(2):807–814. doi: 10.1016/j.expneurol.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siman R, Lin YG, Malthankar-Phatak G, Dong Y. A rapid gene delivery-based mouse model for early-stage Alzheimer disease-type tauopathy. J Neuropathol Exp Neurol. 2013;72(11):1062–1071. doi: 10.1097/NEN.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64(8):1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 102.Oyanagi K, Tsuchiya K, Yamazaki M, Ikeda K. Substantia nigra in progressive supra-nuclear palsy, corticobasal degeneration, and parkinsonism-dementia complex of Guam: specific pathological features. J Neuropathol Exp Neurol. 2001;60(4):393–402. doi: 10.1093/jnen/60.4.393. [DOI] [PubMed] [Google Scholar]
- 103.Spillantini MG, Crowther RA, Kamphorst W, Heutink P, van Swieten JC. Tau pathology in two Dutch families with mutations in the microtubule-binding region of tau. Am J Pathol. 1998;153(5):1359–1363. doi: 10.1016/S0002-9440(10)65721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62(4):389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 105.Klein RL, Dayton RD, Lin WL, Dickson DW. Tau gene transfer, but not alpha-synuclein, induces both progressive dopamine neuron degeneration and rotational behavior in the rat. Neurobiol Dis. 2005;20(1):64–73. doi: 10.1016/j.nbd.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13(3):517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klein RL, Dayton RD, Tatom JB, Diaczynsky CG, Salvatore MF. Tau expression levels from various adeno-associated virus vector serotypes produce graded neurodegenerative disease states. Eur J Neurosci. 2008;27(7):1615–1625. doi: 10.1111/j.1460-9568.2008.06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG, Pramstaller PP, Zanusso G, Ajmar F, Mandich P. Corticobasal degeneration shares a common genetic background with progressive supra-nuclear palsy. Ann Neurol. 2000;47(3):374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 109.Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol. 1994;87(6):545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- 110.Wang DB, Dayton RD, Zweig RM, Klein RL. Transcriptome analysis of a tau over-expression model in rats implicates an early pro-inflammatory response. Exp Neurol. 2010;224(1):197–206. doi: 10.1016/j.expneurol.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ubhi K, Rockenstein E, Doppler E, Mante M, Adame A, Patrick C, Trejo M, Crews L, Paulino A, Moessler H, Masliah E. Neurofibrillary and neurodegenerative pathology in APP-transgenic mice injected with AAV2-mutant TAU: neuroprotective effects of Cerebrolysin. Acta Neuropathol. 2009;117(6):699–712. doi: 10.1007/s00401-009-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Howlett DR, Richardson JC, Austin A, Parsons AA, Bate ST, Davies DC, Gonzalez MI. Cognitive correlates of Abeta deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res. 2004;1017(1–2):130–136. doi: 10.1016/j.brainres.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 113.Richardson JC, Kendal CE, Anderson R, Priest F, Gower E, Soden P, Gray R, Topps S, Howlett DR, Lavender D, Clarke NJ, Barnes JC, Haworth R, Stewart MG, Rupniak HT. Ultrastructural and behavioural changes precede amyloid deposition in a transgenic model of Alzheimer’s disease. Neuroscience. 2003;122(1):213–228. doi: 10.1016/s0306-4522(03)00389-0. [DOI] [PubMed] [Google Scholar]
- 114.Dassie E, Andrews MR, Bensadoun JC, Cacquevel M, Schneider BL, Aebischer P, Wouters FS, Richardson JC, Hussain I, Howlett DR, Spillantini MG, Fawcett JW. Focal expression of adeno-associated viral-mutant tau induces widespread impairment in an APP mouse model. Neurobiol Aging. 2013;34(5):1355–1368. doi: 10.1016/j.neurobiolaging.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 115.Iliev AI, Ganesan S, Bunt G, Wouters FS. Removal of pattern-breaking sequences in microtubule binding repeats produces instantaneous tau aggregation and toxicity. J Biol Chem. 2006;281(48):37195–37204. doi: 10.1074/jbc.M604863200. M604863200 [pii] [DOI] [PubMed] [Google Scholar]
- 116.Ramirez JJ, Poulton WE, Knelson E, Barton C, King MA, Klein RL. Focal expression of mutated tau in entorhinal cortex neurons of rats impairs spatial working memory. Behav Brain Res. 2011;216(1):332–340. doi: 10.1016/j.bbr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jaworski T, Lechat B, Demedts D, Gielis L, Devijver H, Borghgraef P, Duimel H, Verheyen F, Kugler S, Van Leuven F. Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration. Am J Pathol. 2011;179(4):2001–2015. doi: 10.1016/j.ajpath.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawlor PA, Bland RJ, Das P, Price RW, Holloway V, Smithson L, Dicker BL, During MJ, Young D, Golde TE. Novel rat Alzheimer’s disease models based on AAV-mediated gene transfer to selectively increase hippocampal Abeta levels. Mol Neurodegener. 2007;2:11. doi: 10.1186/1750-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vidal R, Frangione B, Rostagno A, Mead S, Revesz T, Plant G, Ghiso J. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399(6738):776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 120.Lewis PA, Piper S, Baker M, Onstead L, Murphy MP, Hardy J, Wang R, McGowan E, Golde TE. Expression of BRI-amyloid beta peptide fusion proteins: a novel method for specific high-level expression of amyloid beta peptides. Biochim Biophys Acta. 2001;1537(1):58–62. doi: 10.1016/s0925-4439(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 121.Drummond ES, Muhling J, Martins RN, Wijaya LK, Ehlert EM, Harvey AR. Pathology associated with AAV mediated expression of beta amyloid or C100 in adult mouse hippocampus and cerebellum. PLoS One. 2013;8(3):e59166. doi: 10.1371/journal.pone.0059166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bolognin S, Blanchard J, Wang X, Basurto-Islas G, Tung YC, Kohlbrenner E, Grundke-Iqbal I, Iqbal K. An experimental rat model of sporadic Alzheimer’s disease and rescue of cognitive impairment with a neurotrophic peptide. Acta Neuropathol. 2012;123(1):133–151. doi: 10.1007/s00401-011-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Pratico D. Adeno-associated virus-mediated brain delivery of 5-lipoxygenase modulates the AD-like phenotype of APP mice. Mol Neurodegener. 2012;7(1):1. doi: 10.1186/1750-1326-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Pratico D. 5-Lipoxygenase gene transfer worsens memory, amyloid, and tau brain pathologies in a mouse model of Alzheimer disease. Ann Neurol. 2012;72(3):442–454. doi: 10.1002/ana.23642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J Histochem Cytochem. 2008;56(12):1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9(10):995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- 127.Tatom JB, Wang DB, Dayton RD, Skalli O, Hutton ML, Dickson DW, Klein RL. Mimicking aspects of frontotemporal lobar degeneration and Lou Gehrig’s disease in rats via TDP-43 overexpression. Mol Ther. 2009;17(4):607–613. doi: 10.1038/mt.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan S, Wang CE, Wei W, Gaertig MA, Lai L, Li S, Li XJ. TDP-43 causes differential pathology in neuronal versus glial cells in the mouse brain. Hum Mol Genet. 2014;23(10):2678–2693. doi: 10.1093/hmg/ddt662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Osinde M, Clavaguera F, May-Nass R, Tolnay M, Dev KK. Lentivirus Tau (P301S) expression in adult amyloid precursor protein (APP)-transgenic mice leads to tangle formation. Neuropathol Appl Neurobiol. 2008;34(5):523–531. doi: 10.1111/j.1365-2990.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 130.Khandelwal PJ, Dumanis SB, Herman AM, Rebeck GW, Moussa CE. Wild type and P301L mutant Tau promote neuro-inflammation and alpha-Synuclein accumulation in lentiviral gene delivery models. Mol Cell Neurosci. 2012;49(1):44–53. doi: 10.1016/j.mcn.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caillierez R, Begard S, Lecolle K, Deramecourt V, Zommer N, Dujardin S, Loyens A, Dufour N, Auregan G, Winderickx J, Hantraye P, Deglon N, Buee L, Colin M. Lentiviral delivery of the human wild-type tau protein mediates a slow and progressive neurodegenerative tau pathology in the rat brain. Mol Ther. 2013;21(7):1358–1368. doi: 10.1038/mt.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hebron ML, Algarzae NK, Lonskaya I, Moussa C. Fractalkine signaling and Tau hyper-phosphorylation are associated with autophagic alterations in lentiviral Tau and Abeta1-42 gene transfer models. Exp Neurol. 2014;251:127–138. doi: 10.1016/j.expneurol.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rebeck GW, Hoe HS, Moussa CE. Beta-amyloid1-42 gene transfer model exhibits intraneuronal amyloid, gliosis, tau phosphorylation, and neuronal loss. J Biol Chem. 2010;285(10):7440–7446. doi: 10.1074/jbc.M109.083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herman AM, Khandelwal PJ, Rebeck GW, Moussa CE. Wild type TDP-43 induces neuro-inflammation and alters APP metabolism in lentiviral gene transfer models. Exp Neurol. 2012;235(1):297–305. doi: 10.1016/j.expneurol.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hebron M, Chen W, Miessau MJ, Lonskaya I, Moussa CE. Parkin reverses TDP-43-induced cell death and failure of amino acid homeostasis. J Neurochem. 2014;129(2):350–361. doi: 10.1111/jnc.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Onorato M, Mulvihill P, Connolly J, Galloway P, Whitehouse P, Perry G. Alteration of neuritic cytoarchitecture in Alzheimer disease. Prog Clin Biol Res. 1989;317:781–789. [PubMed] [Google Scholar]
- 137.Greenamyre JT, Young AB. Excitatory amino acids and Alzheimer’s disease. Neurobiol Aging. 1989;10(5):593–602. doi: 10.1016/0197-4580(89)90143-7. [DOI] [PubMed] [Google Scholar]
- 138.Bassil N, Grossberg GT. Novel regimens and delivery systems in the pharmacological treatment of Alzheimer’s disease. CNS Drugs. 2009;23(4):293–307. doi: 10.2165/00023210-200923040-00003. [DOI] [PubMed] [Google Scholar]
- 139.Rafii MS, Baumann TL, Bakay RA, Ostrove JM, Siffert J, Fleisher AS, Herzog CD, Barba D, Pay M, Salmon DP, Chu Y, Kordower JH, Bishop K, Keator D, Potkin S, Bartus RT. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimer’s Dement. 2014;10:571–581. doi: 10.1016/j.jalz.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 140.Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329(6134):65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 141.Eriksdotter Jonhagen M, Nordberg A, Amberla K, Backman L, Ebendal T, Meyerson B, Olson L, Seiger SM, Theodorsson E, Viitanen M, Winblad B, Wahlund LO. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9(5):246–257. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- 142.Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci U S A. 1999;96(19):10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mandel RJ, Gage FH, Clevenger DG, Spratt SK, Snyder RO, Leff SE. Nerve growth factor expressed in the medial septum following in vivo gene delivery using a recombinant adeno-associated viral vector protects cholinergic neurons from fimbria-fornix lesion-induced degeneration. Exp Neurol. 1999;155(1):59–64. doi: 10.1006/exnr.1998.6961. [DOI] [PubMed] [Google Scholar]
- 144.Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci. 2004;24(36):7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Caccamo D, Katsetos CD, Herman MM, Frankfurter A, Collins VP, Rubinstein LJ. Immunohistochemistry of a spontaneous murine ovarian teratoma with neuro-epithelial differentiation. Neuron-associated beta-tubulin as a marker for primitive neuro-epithelium. Lab Invest. 1989;60(3):390–398. [PubMed] [Google Scholar]
- 146.Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, Leitman E, Binder LI, Brady ST. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging. 2012;33(4):826.e815–826.e830. doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271(51):32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]