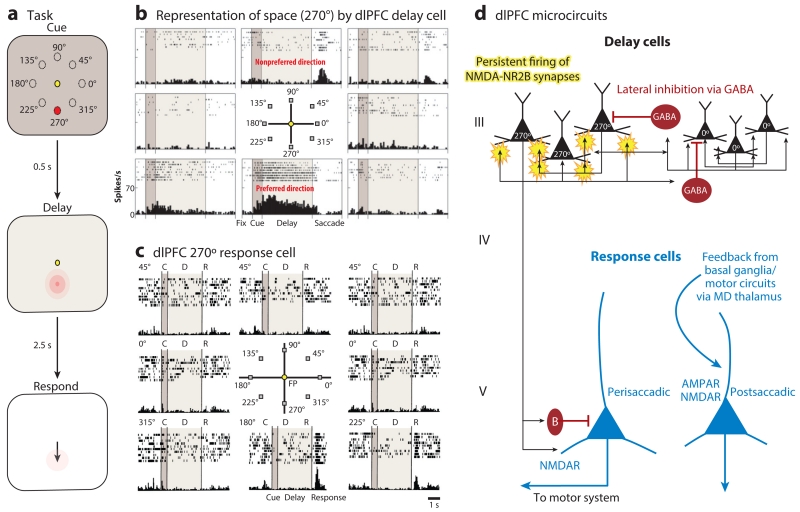
Figure 2.
The delay cells in layer III microcircuits in the dlPFC underlying spatial working memory. (a) Schematic illustration of the ODR task, in which a cue appears briefly (0.5 s) at one of eight locations (e.g., 270°) while the monkey fixates on a central spot. The location must be remembered over a delay period of several seconds until the fixation spot disappears and the monkey can move its eyes to the remembered location for a juice reward. The cued location constantly changes over hundreds of trials, requiring the constant updating of working memory. (b) An example of the firing patterns of a dlPFC delay cell representing the 270° location, the neuron’s preferred direction. This delay cell maintains firing across the delay epoch if the cue had appeared at 270° but not other locations. (c) An example of the firing patterns of a dlPFC response cell that is providing feedback during the eye movement to 270°. Response cells are often inhibited during the delay epoch. (d) The microcircuitry in the primate dlPFC thought to underlie working memory. Microcircuits underlying delay cell firing are thought to reside in deep layer III, the layer that expanded most in primate evolution. Clusters of pyramidal cells with similar preferred directions excite each other to maintain persistent firing across the delay period in the absence of sensory stimulation. This requires glutamate stimulation of NMDAR-NR2B. In contrast, the spatial specificity is refined by lateral inhibition from parvalbumin-containing GABAergic interneurons. In contrast, response cells are thought to reside in layer V. Perisaccadic response cells fire leading up to the motor response, and postsaccadic response cells are thought to carry the corollary discharge feedback that a response has occurred. The postsaccadic response cells are influenced by both NMDAR and AMPAR stimulation. Delay cells likely inhibit response cells during the delay epoch via an inhibitory interneuron. Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; dlPFC, dorsolateral prefrontal cortex; MD, mediodorsal; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; ODR, oculomotor delayed response.