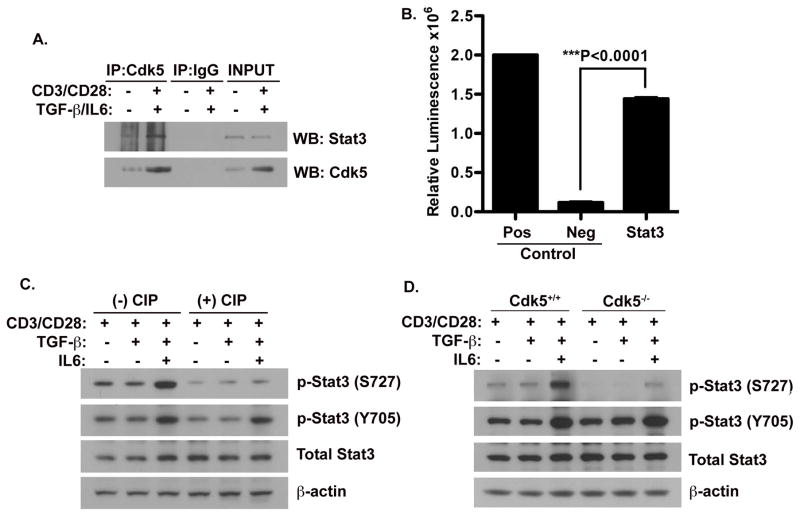
Figure 3. Cdk5 physically interacts with and phosphorylates Stat3 at Serine 727.
A) Protein lysates were isolated from primary T cells before and after TCR activation in the presence of TGF-β, IL-6 and CIP for 72hrs. Samples were then immuno-precipitated with anti-Cdk5 antibody and immunoprecipitates were then probed for the presence of Stat3 and Cdk5. B) A Cdk5-specific in vitro kinase assay was performed to determine the capacity of Cdk5 to phosphorylate Stat3 (the substrate) immunoprecipitated from T cells. Neurofilament-H (NFH) as a substrate, was combined with Cdk5 immunoprecipitates isolated from wild type T cells as a positive control (Pos), whereas Cdk5 immuprecipitates isolated from Cdk5−/− T cells served as the negative control (Neg). C) Western blot analysis of protein lysates isolated from T cells with or without anti-CD3/CD28 activation and treatment with or without TGF-β, IL-6 and CIP for 72hrs. Expression of total Stat3, p-Stat3 (Y705) and p-Stat3 (S727) were determined by Western blot, and β-actin expression was probed as a control. D) Lysates from both Cdk5+/+ T cells and Cdk5−/− T cells stimulated under similar treatment conditions were analyzed by Western blot to determine protein expression of total Stat3, p-Stat3 (Y705), p-Stat3 (S727) and β-actin.