Table 1.
Iron-catalyzed aerobic oxidation of phenyl-substituted 4-benzylpyridines (1).a
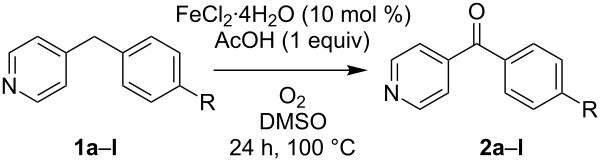 | ||||
| Entry | Substrate | R | Product | Yield (%)b |
| 1 | 1a | H | 2a | 70 |
| 2 | 1b | NH2 | 2b | 55 |
| 3 | 1c | SMe | 2c | 56 |
| 4 | 1d | OMe | 2d | 67 |
| 5 | 1e | Me | 2e | 79 |
| 6 | 1f | I | 2f | 77 |
| 7 | 1g | Br | 2g | 85 |
| 8 | 1h | Cl | 2h | 66 |
| 9 | 1i | F | 2i | 76 |
| 10 | 1j | CO2Et | 2j | 61 |
| 11 | 1k | CN | 2k | 79 |
| 12 | 1l | NO2 | 2l | 60 |
aReactions were performed on a 0.5 mmol scale in 1 mL of solvent using 1 atmosphere of O2 (balloon). bIsolated yields.
