Table 2.
Iron and copper-catalyzed aerobic oxidation of pyridine-substituted 2-benzylpyridines (3).a
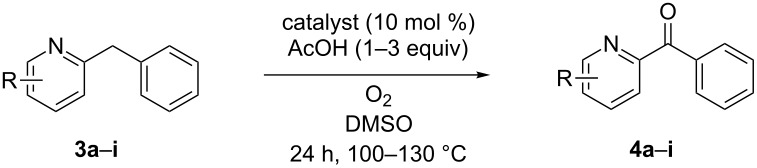 | ||||||
| Entry | Catalyst | Substrate | R | Product | Yield 3 (%)b | Yield 4 (%)b |
| 1 | FeCl2·4H2O | 3a | 5-CN | 4a | 19 | 67 |
| 2 | CuI | 3a | 5-CN | 4a | 18 | 66 |
| 3 | CuI | 3a | 5-CNc | 4a | 0 | 83 |
| 4 | FeCl2·4H2O | 3b | 5-Me | 4b | 9 | 73 |
| 5 | CuI | 3b | 5-Me | 4b | 9 | 82 |
| 6 | CuI | 3b | 5-Mec | 4b | 0 | 72 |
| 7 | FeCl2·4H2O | 3c | 5-OMe | 4c | 65 | 15 |
| 8 | CuI | 3c | 5-OMe | 4c | 66 | 15 |
| 9 | CuI | 3c | 5-OMed,e | 4c | 0 | 65 |
| 10 | FeCl2·4H2O | 3d | 5-CO2Me | 4d | 0 | 69 |
| 11 | CuI | 3d | 5-CO2Me | 4d | 0 | 62 |
| 12 | FeCl2·4H2O | 3e | 5-NHCOMe | 4e | 0 | 64 |
| 13 | CuI | 3e | 5-NHCOMe | 4e | 27 | 56 |
| 14 | CuI | 3e | 5-NHCOMec | 4e | 0 | 91 |
| 15 | FeCl2·4H2O | 3f | 4-Cl | 4f | 0 | 85 |
| 16 | CuI | 3f | 4-Cl | 4f | 8 | 88 |
| 17 | FeCl2·4H2O | 3g | 3-Cl | 4g | 73 | 23 |
| 18 | CuI | 3g | 3-Cl | 4g | 71 | 22 |
| 19 | CuI | 3g | 3-Cld,e | 4g | 0 | 87 |
| 20 | FeCl2·4H2O | 3h | 5-Cl | 4h | 70 | 20 |
| 21 | CuI | 3h | 5-Cl | 4h | 69 | 15 |
| 22 | CuI | 3h | 5-Cld,e | 4h | 0 | 92 |
| 23 | FeCl2·4H2O | 3i | 6-Cl | 4i | 90 | 0 |
| 24 | CuI | 3i | 6-Cl | 4i | 91 | 0 |
| 25 | CuI | 3i | 6-Cle, f | 4i | 0 | 59 |
aReactions were performed on a 0.5 mmol scale in 1 mL of solvent using 1 atmosphere of O2 (balloon). bIsolated yields. c48 h. dAcOH (3 equiv). e130 °C. fTFA (3 equiv).
