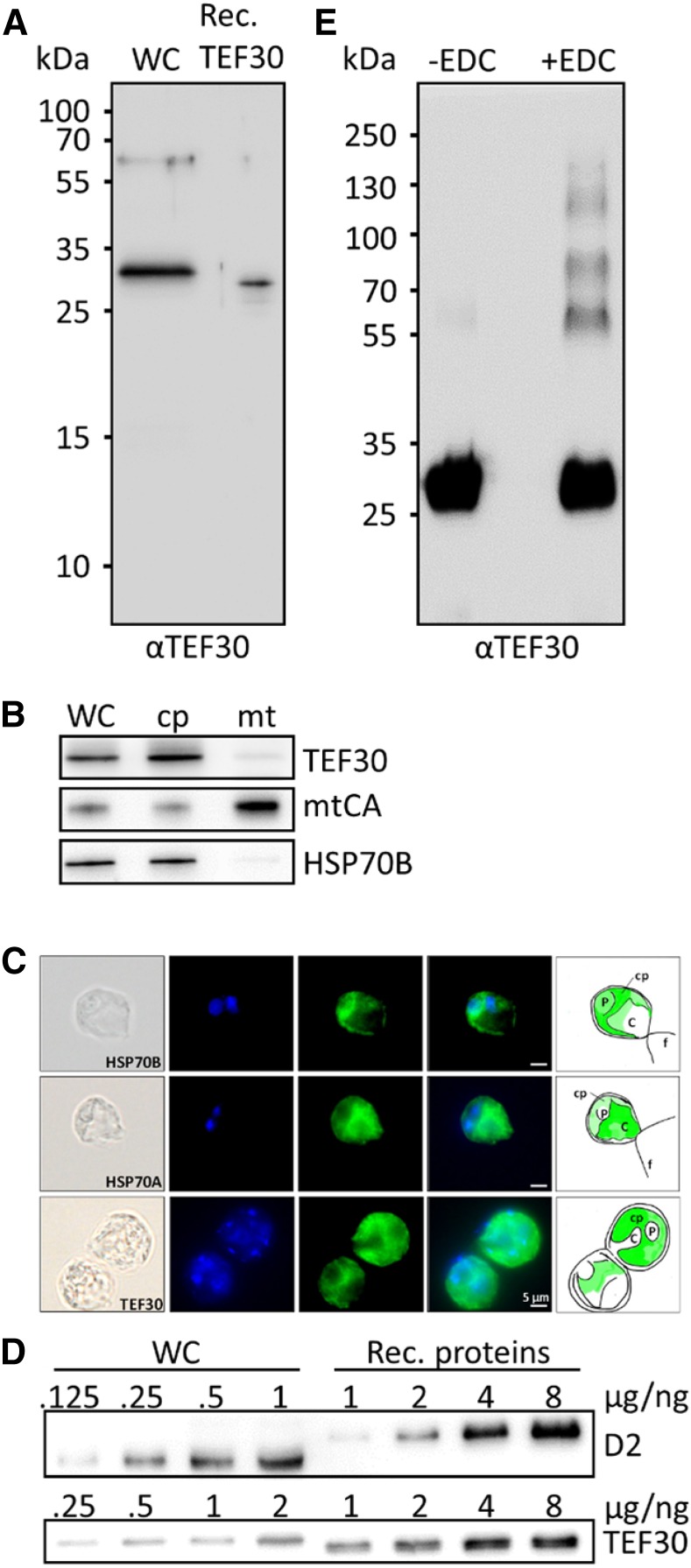
Figure 1.
Antibody characterization and analysis of TEF30 oligomer formation, cellular abundance, and intracellular localization. A, Immunodetection of TEF30 in cw15-325 whole-cell proteins (WC) corresponding to 2 µg of chlorophyll compared with 0.25 ng of recombinant (Rec.) TEF30 protein. B, Subcellular localization of TEF30 by immunoblotting. Ten or 3 µg (depending on the antiserum used) of protein from whole cells, chloroplasts (cp), and mitochondria (mt) isolated from strain cw15-302 was separated by SDS-PAGE and immunodetected with antisera against TEF30, mitochondrial carbonic anhydrase (mtCA), and stromal HSP70B. C, Microscopy images taken from strain cw15-325. Shown from left to right are differential interference contrast images, 4′,6-diamino-phenylindole (DAPI) staining, immunofluorescence, merge of DAPI and immunofluorescence, and a schematic drawing that combines information from the images. Antisera used for immunofluorescence were against HSP70B and HSP70A as markers for stroma and cytosol, respectively, and against TEF30. C, Cytosol; f, flagella; P, pyrenoid. Immunofluorescence signal is shown in green. D, One to 8 ng of recombinant proteins TEF30 (purified from E. coli) and D2 (purchased from Agrisera) was separated by SDS-PAGE together with cw15-325 whole-cell proteins corresponding to 0.125 to 2 µg of chlorophyll and immunodetected with antibodies against TEF30 and D2. E, Recombinant TEF30 protein was incubated with EDC/N-hydroxysuccinimide, separated on an SDS-polyacrylamide gel, and immunodetected with antibodies against TEF30.