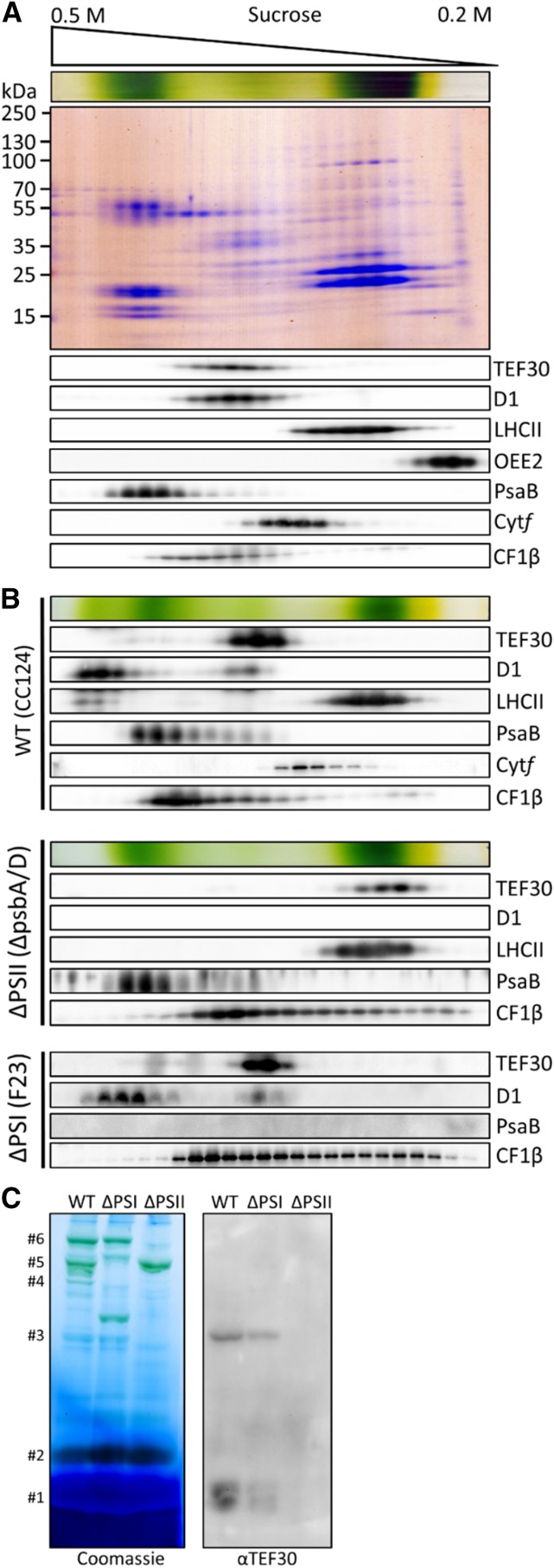
Figure 4.
Comigration of TEF30 with PSII core subunits as revealed by Suc density gradient centrifugation and blue native (BN)-PAGE. A, Thylakoid membranes from strain cw15-325 (1 mg chlorophyll mL−1) were solubilized with 1% β-dodecyl maltoside (β-DDM) and separated on a linear 0.2 to 0.5 m Suc gradient at 205,000g for 16 h. Proteins were collected in 25 fractions, separated by SDS-PAGE, and detected with Colloidal Blue staining (top gel) or immunologically with the antisera indicated (bottom gels). B, Analysis of thylakoid membrane protein complexes by Suc density gradient centrifugation as described in A on wild-type (WT) strain cc124, PSI mutant F23, and PSII mutant ΔpsbA/D. C, Analysis of thylakoid membrane protein complexes by BN-PAGE. Thylakoids from wild-type strain cc124, PSI mutant F23, and PSII mutant nac2 were solubilized with 1% β-DDM, and proteins corresponding to 5 µg of chlorophyll were separated on a 5% to 15% polyacrylamide BN gradient gel, followed by Coomassie Blue staining (left gel) and immunodetection with antibodies against TEF30 (right gel). Protein bands were assigned to LHCII monomers (#1), LHCII trimers (#2), PSII monomers (#3), PSII dimers (#4), PSI supercomplexes (#5), and PSII supercomplexes (#6) according to Järvi et al. (2011).