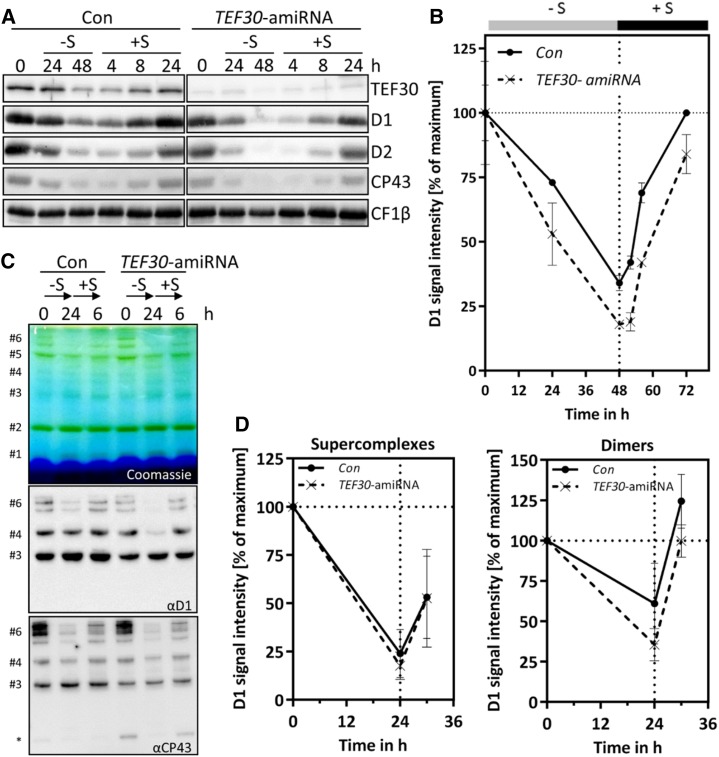
Figure 7.
PSII de novo synthesis is not impaired in TEF30-amiRNA strains. A, TEF30-amiRNA and control (Con) cells in the cw15-325 background were grown in TAP-NH4 at approximately 30 µmol photons m−2 s−1 to the exponential phase. Cells were then harvested, washed, and resuspended in TAP medium lacking sulfur (–S). After 48 h under –S conditions, cells were shifted back to regular TAP medium (+S) for another 24 h. Whole-cell proteins from samples taken during this time course were separated by SDS-PAGE, and PSII polypeptide abundance was measured via immunoblotting using the antibodies indicated and CF1β as a loading control. B, D1 signal intensities from A were quantified using the FUSIONCapt Advance program. Signals were normalized to the initial D1 levels in each strain. Error bars represent sd; n = 2. C, Sulfur depletion was done as in A, but time in –S and +S conditions was reduced to 24 and 6 h, respectively. Thylakoids were solubilized with 1% α-dodecyl maltoside (α-DDM), and proteins corresponding to 5 µg of chlorophyll were separated on a 5% to 15% polyacrylamide BN gradient gel, followed by Coomassie Blue staining (top gel) and immunodetection with antibodies against D1 and CP43 (bottom gels). Protein bands were assigned as in Figure 4C; the asterisk designates free CP43. D, D1 signal intensities from protein bands #6 (PSII supercomplexes) and #4 (PSII dimers) from C were quantified using the FUSIONCapt Advance program. Signals were normalized to the initial D1 levels in each strain. Error bars represent sd; n = 4.