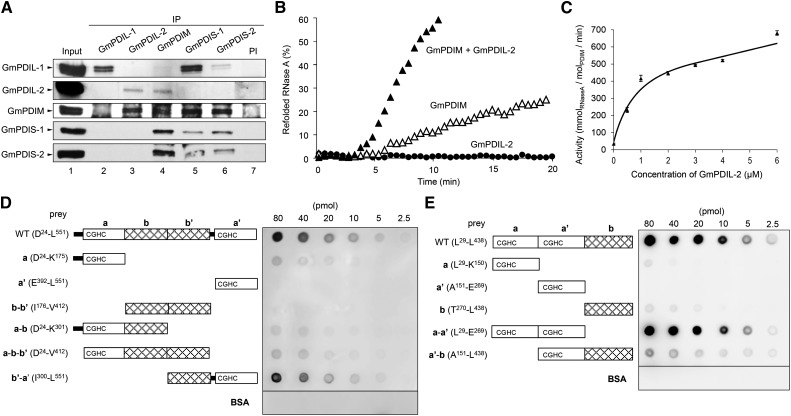
Figure 5.
GmPDIL-2 and GmPDIM form a complex, and synergistically refold RNase A using disulfide bonds produced by GmERO1a. A, Detection of ER oxidoreductase complexes. IP from the cotyledon extract was carried out with each anti-ER oxidoreductase serum or PI. Immunoprecipitates and the cotyledon extract (lane 1) were analyzed by western blot with each anti-ER oxidoreductase serum. B, Effects of the addition of GmPDIL-2 to GmPDIM on the refolding of RNase A. Reduced and denatured RNase A (8 μm) was incubated with 3 μm ER oxidoreductases in the presence of 1 μm GmERO1a. C, GmPDIL-2 concentration dependence of the refolding activity in the presence of 1 μm GmERO1a and 1 μm GmPDIM. Data are represented as mean ± se of n = 3. D, Far-western blot analysis of the association of the GmPDIL-2 (WT) or GmPDIL-2 domain fragment with GmPDIM. Indicated amounts of GmPDIL-2, each GmPDIL-2 domain fragment, or BSA (prey) were dot-blotted and incubated with GmPDIM. Bound GmPDIM was immunostained. E, For GmPDIM (WT), each GmPDIM domain fragment or BSA (prey) was dot-blotted and incubated with GmPDIL-2. Bound GmPDIL-2 was immunostained. BSA, bovine serum albumin; IP, immunoprecipitation; PI, preimmune serum; WT, wild type.