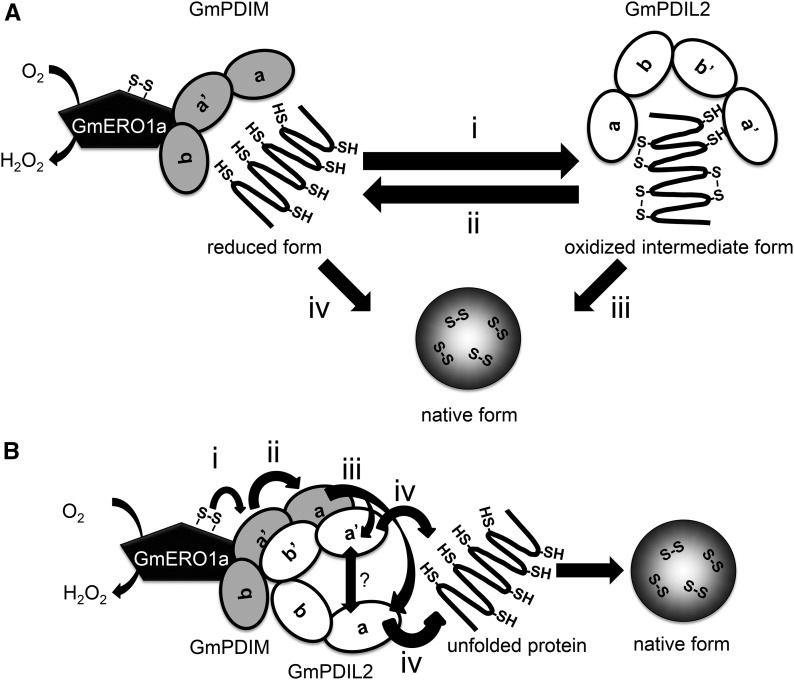
Figure 9.
Models of cooperation of GmPDIM and GmPDIL-2. A, Role-sharing model of GmPDIM and GmPDIL-2. GmPDIM oxidized by GmERO1a introduces transient disulfide bonds in an unfolded substrate protein (i). Transient disulfide bonds in the substrate protein are reduced by the reduced form of GmPDIL-2 (ii). Reduced thiols are rearranged into native disulfide bonds, mainly by GmPDIL-2 (iii), but partially by GmPDIM (iv). B, model of oxidative relays from GmERO1a to GmPDIM, GmPDIL-2, and substrate. GmERO1a oxidizes the active center in the a′ domain of GmPDIM (i). The formed disulfide bond is transferred sequentially from the a′ domain of GmPDIM to the a domain of GmPDIM (ii), either the a or a′ domain of GmPDIL-2 (iii), and substrate (iv). Transferring of a disulfide bond between the a and a′ domains of GmPDIL-2 is unclear. Both a and a′ domains of GmPDIM and both b′ and a′ domains of GmPDIL-2 are essential for the association of GmPDIM and GmPDIL-2.