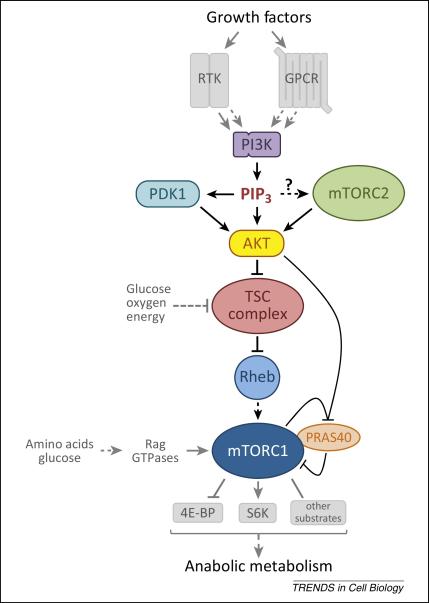
Figure 1.
The primary pathway through which class I PI3K activates mTORC1. Growth factors (including hormones, cytokines, and chemokines) activate RTKs or GPCRs which, through a variety of mechanisms, activate PI3K. PI3K generates PIP3 which specifically binds Akt and PDK1 promoting the phosphorylation and activation of Akt by PDK1. Phosphorylation of Akt by mTORC2 boosts its activity several-fold and mTORC2 activation is at least partially PI3K-dependent. Akt inhibits the TSC complex, the specific GAP for the small GTPase Rheb, through multi-site phosphorylation of the TSC2 subunit. This relieves inhibition of Rheb, allowing it to become activate and stimulate mTORC1 kinase activity. Once mTORC1 is activated by Rheb, the simultaneous phosphorylation of its inhibitory subunit PRAS40 by Akt and mTORC1 itself causes PRAS40 to dissociate from mTORC1. This is thought to increase substrate access to the complex. Glucose, oxygen, and energy levels are also sensed upstream of the TSC complex. Amino acids (and glucose) are sensed upstream of mTORC1 via pathways that regulate the Rag GTPases, which do not directly activate mTORC1 but serve to bring it into proximity with Rheb in cells. mTORC1 directly phosphorylates numerous substrates including S6K and 4E-BP which mediate its control of anabolic metabolism, cellular growth, and proliferation.