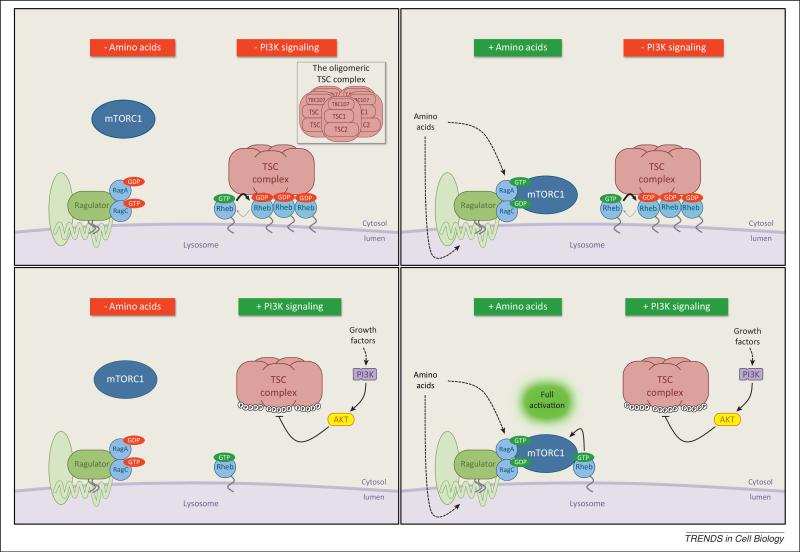
Figure 2.
A working model of mTORC1 activation by PI3K signaling and amino acids at the lysosome. Proper activation of mTORC1 requires both the Rag and Rheb GTPases, which are activated by amino acids and growth factors respectively. These signals are integrated at the cytoplasmic face of the lysosomal membrane where mTORC1 is activated. In the absence of amino acids, mTORC1 localizes away from the lysosome. Amino acid signaling activates the Rag GTPases which directly recruit mTORC1 to the lysosome but do not stimulate its kinase activity. The Rags, which function as heterodimers consisting of A or B with C or D isoforms, associate with the lysosome through their interaction with the membrane-tethered Ragulator complex. The Ragulator also associates with integral membrane proteins and complexes of the lysosome that participate in amino acid sensing (light green, unlabeled). A variety of other amino acid-sensitive regulators of mTORC1 have been identified but are not shown. In the absence of growth factors, the TSC complex, which is the GAP for Rheb, localizes to the lysosome in a Rheb-dependent manner and keeps Rheb inactive. Stimulation with growth factors leads to the PI3K-dependent activation of Akt with then phosphorylates TSC2 on multiple sites within the TSC complex. These phosphorylation events induce dissociation of the intact TSC complex from the lysosome and Rheb, allowing Rheb to become active and directly simulate mTORC1. Hence amino acid signaling to the Rags and growth factor-PI3K signaling to Rheb represent parallel, independent inputs that are each necessary but no sufficient for full activation of mTORC1. The TSC complex is a large oligomeric structure, composed of approximately five copies of each subunit (TSC2, TSC2, and TBC1D7) (inset box). In addition to functioning as a GAP for Rheb and converting it to its inactive GDP-bound state, the TSC complex might also prevent Rheb from activating mTORC1 by sequestering its GDP-bound form. Basal activation of either the Rags or Rheb may result in incomplete effects of amino acid or growth factor starvations on mTORC1 activation.