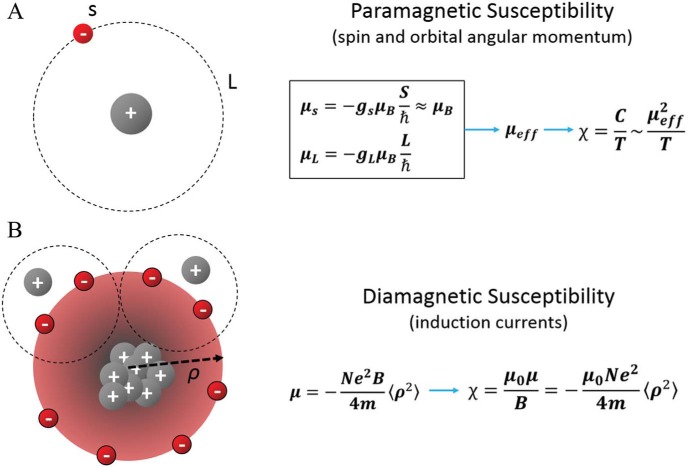
Figure 2.
Atomic origin of paramagnetic and diamagnetic susceptibility. (A) Paramagnetic susceptibility originates primarily from spin and orbital angular momentum induced magnetic moments (μs and μL, respectively) of electrons. Electrons can be found in these quantized momentum levels following the Boltzmann distribution, resulting in an expected magnetic moment μeff and an paramagnetic susceptibility inversely proportional to temperature. (B) Diamagnetic susceptibility originates from the precession of orbital electrons about the applied external magnetic field. The precession of electrons is modeled as a circular current, which generates a secondly field opposing the applied magnetic field. Thus, the resulting susceptibility is diamagnetic.