Abstract
A 61-year-old female presented with eosinophilic pneumonia accompanied by bronchial asthma. She was finally diagnosed with allergic bronchopulmonary mycosis (ABPM) due to co-infection with Aspergillus fumigatus and Schizophyllum commune detected by genetic analysis of the plug and from cultures.
Keywords: Allergic broncho-pulmonary mycosis (ABPM), Aspergillus fumigatus, Bronchial asthma, Eosinophilic pneumonia, Schizophyllum commune, Next-generation sequencer
Case report
A 61-year-old female who had never smoked was referred to Osaka University Hospital with a productive cough, shortness of breath, and wheezing for 5 years. She had a high eosinophil range of 6–20% (normal, 2–4%) and transient recurrent infiltrates on serial chest X-rays. Eosinophilic pneumonia with bronchial asthma had been diagnosed and treatment with oral prednisolone resulted in the remission of symptoms for 2–3 weeks at a time.
The patient complained of up to 10 mL of sputum per day which was purulent, malodorous and sometimes formed mucoid plugs. She was prescribed with inhaled corticosteroids and long-acting beta-agonists. She was found to have bilateral coarse crepitations with biphasic polyphonic rhonchi on pulmonary examination. Blood cell analysis revealed a total leukocyte count of 7240 cells/μL; with 44.7% polymorphs, 36.8% lymphocytes, 5.5% monocytes, 11.9% eosinophils, and 1.1% basophils. The absolute eosinophil count was elevated at 861/μL. Serum biochemistry findings were unremarkable, and Aspergillus spp. IgG antibody was negative. Total IgE was within the normal range (70.36 IU/mL), but specific IgE for Aspergillus spp. was found to be positive.
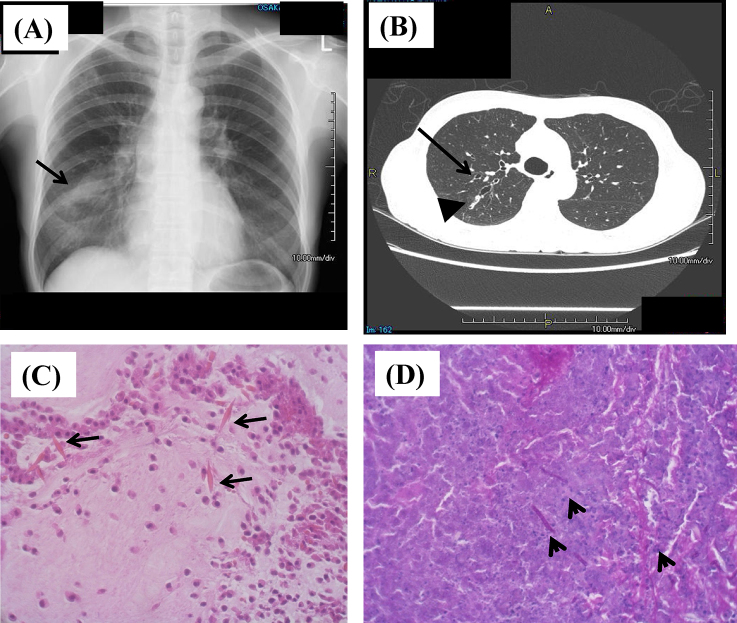
A chest X-ray showed infiltrates in the right lower lung (Fig. 1A), and computed tomography revealed bilateral dilation of the central bronchi in the right upper lobe (Fig. 1B). The involved bronchi contained hyperattenuating mucus. Bilateral central bronchiectasis was diagnosed. The patient was further investigated because saccular bronchiectasis is a feature of allergic bronchopulmonary aspergillosis (ABPA). Direct analysis of sputum samples using random sequencing (shotgun sequence) using a next-generation sequencer revealed fungal DNA. Aspergillus fumigatus was dominant and four other fungal sequences including Schizophyllum commune were found (Table 1).
Fig. 1.
Chest images and pathological findings of mucus plug from the patient. (A) X-ray image shows infiltration shadows (arrow) that moved in each period. (B) Computed tomography image shows proximal bronchiectasis (arrow) and mucus plug (arrowhead). (C) Hematoxylin–eosin staining shows Charcot–Leyden crystals (arrows) with clusters of eosinophils. (D). Periodic acid–Schiff's stain shows hyphae (arrowheads) indicating fungal infection.
Table 1.
Candidate fungus detected by shotgun sequence.
| Query | BLAST suggestion | |
|---|---|---|
| Single candidate | 1 | Aspergillus fumigatus |
| 2 | Aspergillus fumigatus | |
| 3 | Aspergillus fumigatus | |
| 4 | Schizophyllum commune | |
| Multiple candidate | 5 | Aspergillus fumigatus, Penicillium, Talaromyces |
| 6 | Aspergillus fumigatus, Penicillium, Talaromyces, Chaetomium | |
| 7 | Unknown Penicillium, Dunaliella | |
| 8 | Aspergillus fumigatus, Aspergillus terreus | |
| 9 | Penicillium solitum, Aspergillus oryzae | |
| 10 | Paecilomyces, Aspergillus fumigatus, Neosartorya | |
| 11 | Paecilomyces, Aspergillus fumigatus, Neosartorya | |
| 12 | Paecilomyces, Aspergillus fumigatus, Neosartorya | |
| 13 | Cryptocercus, Metainae, Hispanognatha, Antillognata, Allende | |
| 14 | Penicillium solitum, Aspergillus oryzae | |
| 15 | Unknown Penicillium, Unknown Aspergillus, Poria | |
| 16 | Penicillium chrysogenum, Aspergillus niger, Poria cocos, Aspergillus oryzae | |
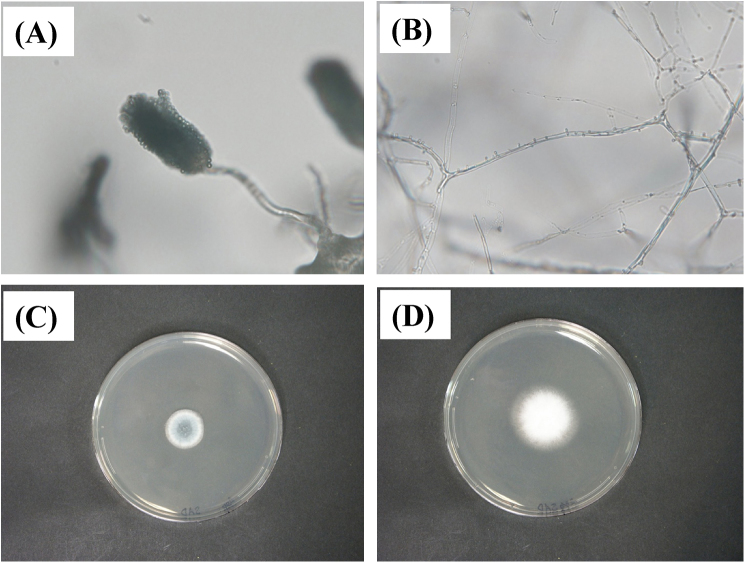
Microscopic assessment of sputum was negative for acid-fast bacilli (AFB) and other bacteria. However, Charcot–Leyden crystals that are generated through the breakdown of eosinophils and hyphae were identified (Fig. 1C and D). Direct microscopy of KOH wet mounts of three consecutive sputum specimens and expectorated sputum/mucoid plugs revealed two types of septate and branching hyphae (Fig. 2A and B). Homogenized sputum and mucoid plugs inoculated onto Sabouraud's glucose agar (SGA) developed green and white colonies with a wooly texture after 4 days at 2 °C (Fig. 2C and D).
Fig. 2.
Aspergillus fumigatus and Schizophyllum commune isolated from mucus plug. (A) Morphologically characterized A. fumigatus. (B) Cultured S. commune isolate shows hyaline, septate hyphae with clamp connections and spicules (400×). (C) Granular to cottony, green-gray Aspergillus fumigatus colonies with apron at margin. (D) White, wooly colonies of S. commune isolated from sputum and inoculated onto SGA supplemented with benomyl after 4 days of incubation at 28 °C. Slide (A and B) and SGA (C and D) cultures.
One of the fungi was morphologically identified as A. fumigatus (Fig. 2A and C), but the other (Fig. 2B and D) remained obscure. Therefore, DNA was extracted and the internal transcribed spacer region (ITS) and the D1/D2 region of large subunit rRNA gene were amplified by PCR. Direct sequencing of the PCR products showed that the sequences of the ITS and D1/D2 regions were 100% similar to those of various S. commune strains, such as IFM 46097. The diagnosis of allergic bronchopulmonary mycosis (ABPM) due to co-infection with A. fumigatus and S. commune was made.
The susceptibility of the isolated A. fumigatus and S. commune strains to antifungal drugs (minimum inhibitory concentration; MIC) was as follows: amphotericin B (1 and 0.5 μg/mL), 5-fluorocytosine (64 and 0.25 μg/mL), fluconazole (>64 and 4 μg/mL), itraconazole (0.12 and 0.25 μg/mL), voriconazole (0.5 and 0.12 μg/mL), and micofungin (0.03 and >16 μg/mL), respectively.
The administration of oral itraconazole and prednisolone, improved the symptoms in this patient.
Discussion
The form of pulmonary hypersensitivity known as allergic bronchopulmonary mycosis (ABPM) is generally diagnosed using the criteria by Rosenberg et al. [1]. The most common etiological agent is A. fumigatus, but other Aspergillus species and fungi can also be responsible [2], [3], [4], [5].
S. commune (“suehirotake” in Japanese), is a widely distributed fleshy fungus that is often found on decaying organic matter, especially rotting wood. It belongs to the Phylum Basidiomycota, Subphylum Agaricomycotina, Order Agaricales that contains fungi that are colloquially known as mushrooms [2]. Notwithstanding the worldwide distribution of S. commune, it was first identified as a human pathogen when Kligman isolated it from a patient with onychomycosis in 1950 [6]. Since then, it has been increasingly reported as a cause of ABPM and other allergy-related bronchopulmonary and sinus diseases. Here, we describe ABPM that was caused by A. fumigatus and S. commune co-infection. This condition was diagnosed morphologically and genetically using a next-generation sequencer.
When ABPM is considered in a patient with mucoid impaction of the bronchi (MIB) [7], the etiology is usually based on cultural criteria. Molecular analyses have become more accurate for identifying environmental fungi, but when unknown samples or plural eumycetes are cultured from bronchial materials of MIB patients, meticulous attention to detail is needed to diagnose ABPM [3].
S. commune has been identified as an etiological agent of ABPM as well as pulmonary fungal balls. This fungus is characterized by clamp connections, hyphal spicules and the formation of basidiocarps with basidiospores. Phenotypic identification is usually confirmed by sequencing the ITS region. To date, ABPM and pulmonary fungal balls due to S. commune have been found predominantly in Japan and North America. Of the 71 globally reported patients harboring S. commune, 45 (63%) had bronchopulmonary disease, 22 (31%) had sinusitis and four had extrapulmonary disease. The total number of bronchopulmonary disease and sinusitis was 67 (94%), indicating that the respiratory tract is the primary target of S. commune [2], [4], [5]. Chowdhary et al. reported that ABPM in one of 19 patients from Japan was caused by concomitant S. commune and A. fumigates colonization, as in our patient [2], [5].
Greer and Bolanos experimentally demonstrated the pathogenic potential of S. commune in Swiss white mice using a fragmented hyphal inoculum and an intraperitoneal route of infection [8]. This fungus was more pathogenic to weaning mice than to adults. In this model, cortisone or mucin or both rendered the mice much more highly susceptible, causing a 75% fatality rate with histological lesions in the lungs, lymph nodes, liver and subcutaneous tissue.
We detected sequences that were more similar to those of S. commune using a next-generation sequencer and confirmed the PCR-amplified genetic sequence. Our results suggest that these tests are valuable when patients present with infections caused by unknown fungi. Although high-throughput next-generation sequencing technologies might be difficult to apply during routine clinical diagnosis, they have potential for clinical genomic studies, as they can vastly exceed the data output of the most sophisticated capillary sequencers based on the Sanger method [9].
With bacteremia, detection and confirmation of pathogens can be done quickly in patients using metagenomic sequencing can be used along with blood cultures [10]. Specific fungal pathogens can be directly detected and genetically analyzed similarly within 1 day, whereas routine fungal cultures may require weeks to detect and identification certain pathogens. A more rapid approach would benefit patients.
The role of antifungal therapy in ABPM and pulmonary fungal balls due to S. commune remains unclear because of the rarity of reports about these clinical entities and consequently limited experience with their chemotherapeutic management. Chowdhary et al. reported that oral and inhaled corticosteroids improved symptoms in one patient with ABPM, whereas itraconazole improved pulmonary fungal ball in a patient with diabetes [2]. In vitro susceptibility tests of clinical S. commune isolates from both of these patients revealed low MICs for amphotericin B, caspofungin, micafungin, anidulafungin and all of the azoles. Information about the antifungal sensitivity of S. commune in vitro is, however, scarce. One study found that five test clinical isolates of S. commune were susceptible to itraconazole, voriconazole and amphotericin B [11]. Further studies of the susceptibility of a large number of S. commune isolates to antifungal agents in vitro are required to ensure appropriate and effective antifungal therapy against these emerging pathogens, and long-term follow-up is required to evaluate the therapeutic value of antifungal agents.
In conclusion, we describe a patient who had been misdiagnosed with eosinophilic pneumonia and bronchial asthma but, in fact, had ABPM due to co-infection with A. fumigatus and S. commune. The pathogens were detected morphologically and by genetic sequencing. Anti-fungal therapy improved the symptoms and X-ray findings in this patient, but further study of the pathogenesis of ABPM and S commune is needed.
Conflicts of interest
None.
Consent
Informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. This study was approved by the ethics committee of Osaka University (11160-3).
Acknowledgements
The authors thank Dr. Nori Yoshioka and Isao Nishi (Osaka University) for support with the microbiological examinations, and this study was supported by a Grant-in-Aid for Scientific Research 235911510 (to M.S.) from the Japanese Society for the Promotion of Science.
References
- 1.Rosenberg M., Patterson R., Mintzer R., Cooper B.J., Roberts M., Harris K.E. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977;86:405–414. doi: 10.7326/0003-4819-86-4-405. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhary A., Randhawa H.S., Gaur S.N., Agarwal K., Kathuria S., Roy P. Schizophyllum commune as an emerging fungal pathogen: a review and report of two cases. Mycoses. 2013;56:1–10. doi: 10.1111/j.1439-0507.2012.02190.x. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa H., Fujimura M., Takeuchi Y., Makimura K., Satoh K. The definitive diagnostic process and successful treatment for ABPM caused by Schizophyllum commune: a report of two cases. Allergol Int. 2012;61:163–169. doi: 10.2332/allergolint.11-CR-0325. [DOI] [PubMed] [Google Scholar]
- 4.Kamei K., Unno H., Nagao K., Kuriyama T., Nishimura K., Miyaji M. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin Infect Dis. 1994;18:305–309. doi: 10.1093/clinids/18.3.305. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa H., Ogawa H., Fujimura M., Takeuchi Y., Makimura K. Two cases of Schizophyllum asthma: is this a new clinical entity or a precursor of ABPM? Pulm Pharmacol Ther. 2011;24:559–562. doi: 10.1016/j.pupt.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Kligman A.M. A basidiomycete probably causing onychomycosis. J Invest Dermatol. 1950;14:67–70. doi: 10.1038/jid.1950.10. [DOI] [PubMed] [Google Scholar]
- 7.Amitani R., Nishimura K., Niimi A., Kobayashi H., Nawada R., Murayama T. Bronchial mucoid impaction due to the monokaryotic mycelium of Schizophyllum commune. Clin Infect Dis. 1996;22:146–148. doi: 10.1093/clinids/22.1.146. [DOI] [PubMed] [Google Scholar]
- 8.Greer D.L., Bolanos B. Pathogenic potential of Schizophyllum commune isolated from a human case. Res Commun Chem Pathol Pharmacol. 1972;3:233–244. [PubMed] [Google Scholar]
- 9.Pareek C.S., Smoczynski R., Tretyn A. Sequencing technologies and genome sequencing. J Appl Genet. 2011;52:413–435. doi: 10.1007/s13353-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki M., Gotoh K., Nakamura S., Akeda Y., Yoshii T., Miyaguchi S. Fatal sepsis caused by an unusual Klebsiella species that was misidentified by an automated identification system. J Med Microbiol. 2013;62(801–803):801–803. doi: 10.1099/jmm.0.051334-0. [DOI] [PubMed] [Google Scholar]
- 11.González G.M., Sutton D.A., Thompson E., Tijerina R., Rinaldi M.G. In vitro activities of approved and investigational antifungal agents against 44 clinical isolates of basidiomycetous fungi. Antimicrob Agents Chemother. 2001;45:633–635. doi: 10.1128/AAC.45.2.633-635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]