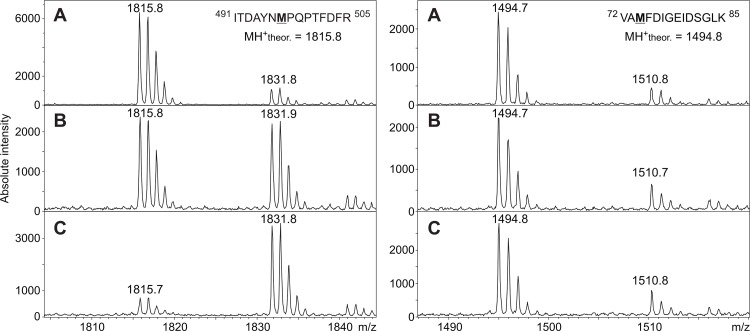
Fig 2. Mass spectrometric identification of methionine residues oxidized by H2O2 during TmPOx inactivation.
MALDI MS spectra were measured for unaffected POx (A), for POx inactivated during D-glucose oxidation (B) or for POx inactivated by endogenous H2O2 (C). The selected MALDI spectra in the left panel illustrate that Met497 of the tryptic peptide ITDAYNMPQPTFDFR with a theoretical MH+ of 1815.8 was extensively oxidised in TmPOx inactivated either during substrate turnover (B, left panel) or by H2O2 treatment (C, left panel). In contrast, some methionine residues were found not to be oxidised during TmPOx inactivation as shown for Met74 of the peptide VAMFDIGEIDSGLK having a MH+ of 1494.8 (right panel). The small signals at m/z 1510.8 are related to the oxidized form of the peptide generated due to the presence of air oxygen.