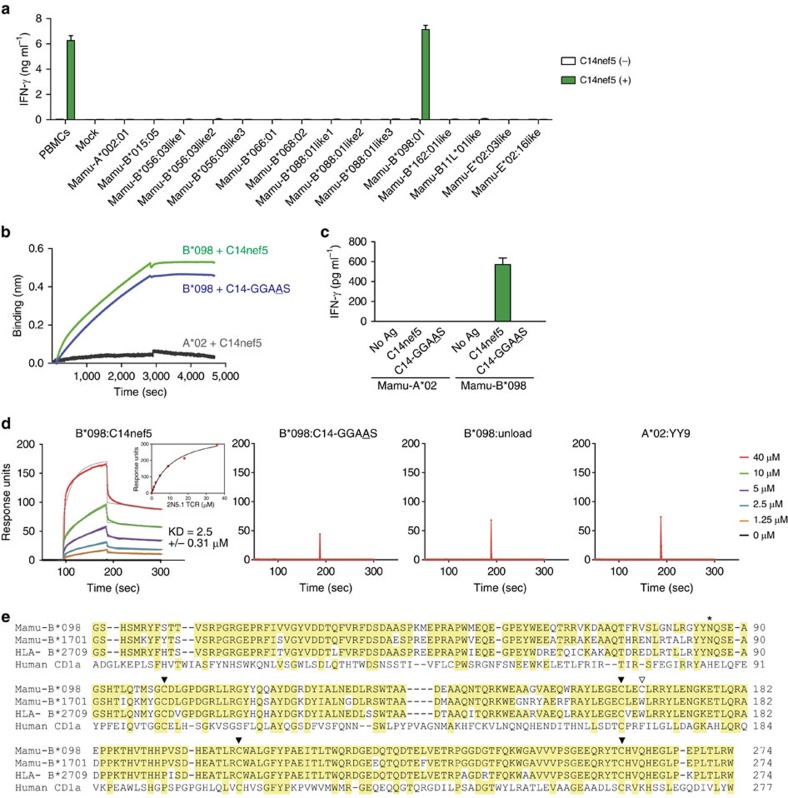
Figure 1. Identification of Mamu-B*098 as a lipopeptide Ag-presenting molecule.
(a) LLC-MK2 cells transfected with each of the rhesus MHC class I genes were tested for their ability to present C14nef5 to 2N5.1. Only LLC-MK2 cells expressing Mamu-B*098 stimulated 2N5.1 to produce IFN-γ in the presence of C14nef5. PBMCs obtained from a donor with the potential to present C14nef5 to 2N5.1 were used as a positive control. Experiments were performed in triplicate. Mean values with s.e.m. are shown. (b) The binding of lipopeptide Ags to recombinant β2m-linked Mamu-B*098 and Mamu-A*02 proteins was monitored by biolayer interferometry. Representative data from three independent experiments are shown. Mamu-A*02, a rhesus MHC class I allele known to bind 9-mer peptides, was used as a negative control. (c) Plate-coated MHC class I molecules were tested for their ability to present lipopeptide Ags to 2N5.1. The Ag-specific T-cell response was assessed by measuring the amount of IFN-γ released into the culture medium. Mean values with s.e.m. are shown. (d) The high-affinity interaction of the 2N5.1 TCR with C14nef5-loaded Mamu-B*098 was demonstrated by SPR-binding assays, whereas no interaction was detected with C14-GGAAS-loaded and unloaded Mamu-B*098 molecules, as well as YY9 peptide-loaded Mamu-A*02 molecules. The original data (coloured as indicated) are depicted with the curve fit (grey) overlaid. The steady-state affinity plot (inset) is also shown. Three independent experiments were performed and mean equilibrium-dissociation constant (KD) values ±s.d. are shown. (e) Amino acid sequences of Mamu-B*098, HLA-B*27:09, Mamu-B*17:01 and human CD1a are shown. The α1 and α2 domains of Mamu-B*098 exhibit 81.1%, 80.6% and 20.6% sequence homology to the corresponding domains of HLA-B27, Mamu-B*17 and human CD1a, respectively. Solid and open triangles indicate paired and unpaired cysteine residues, respectively. The residue for N-glycosylation is indicated with an asterisk.