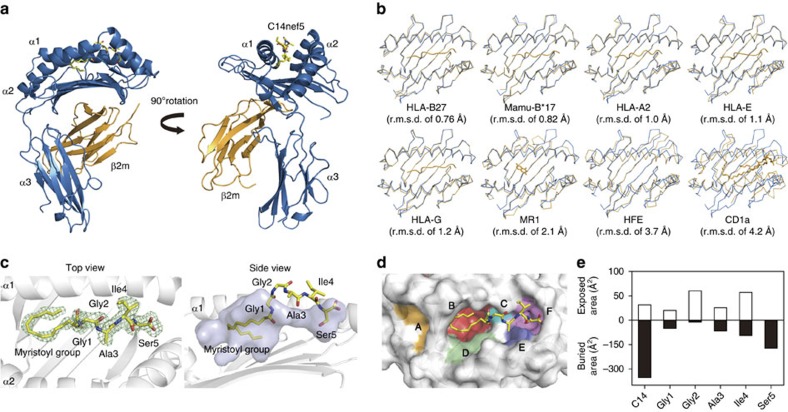
Figure 2. The overall structure of the Mamu-B*098 complex.
(a) Two views of the trimer complex of the ectodomain of Mamu-B*098 heavy chains (blue), β2m (orange) and C14nef5 (yellow) are shown. (b) Superimposed images of the α1 and α2 domains of Mamu-B*098 (blue) with those of MHC class I and MHC class I-like molecules (orange) are shown. (c) C14nef5 binding to Mamu-B*098 is demonstrated by a 2Fo-Fc map (green mesh) contoured at 0.8σ (left). The bound lipopeptide accommodated in the semi-transparent Ag-binding cavity is shown (right). (d) The surface of the Ag-binding groove with pockets A through F is shown. (e) Solvent accessible surfaces of C14nef5 captured by Mamu-B*098 are shown for the myristoyl group and each of the amino acid residues. Filled and unfilled bars indicate buried and exposed areas, respectively.