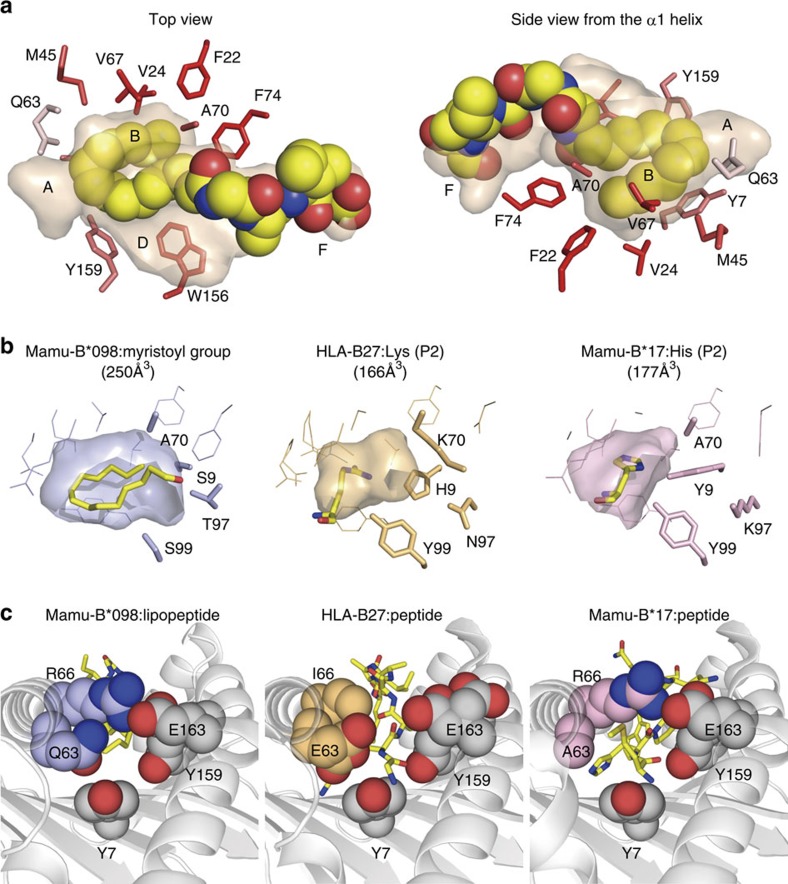
Figure 3. Interactions with the acyl chain of the lipopeptide.
(a) C14nef5 is shown in yellow as a space-filling model with the semi-transparent Ag-binding groove of Mamu-B*098. The side chains of residues surrounding the acyl chain are shown in red. The intensity of the red colour correlates with the degree of hydrophobicity. (b) B pockets of Mamu-B*098 (blue), HLA-B27 (light orange) and Mamu-B*17 (pink) are shown as the semi-transparent groove and the side chains (lines) with emphasis on the non-bulky side chains (Ser9, Ala70, Thr97 and Ser99) of Mamu-B*098. C14 acyl chain or amino acid residues accommodated in each B pocket are displayed as yellow sticks. (c) A view from the A pocket points to a narrower channel between the A and B pockets in Mamu-B*098 (left) than in HLA-B27 (middle) and Mamu-B*17 (right). Key residues are presented as a space-filling model.