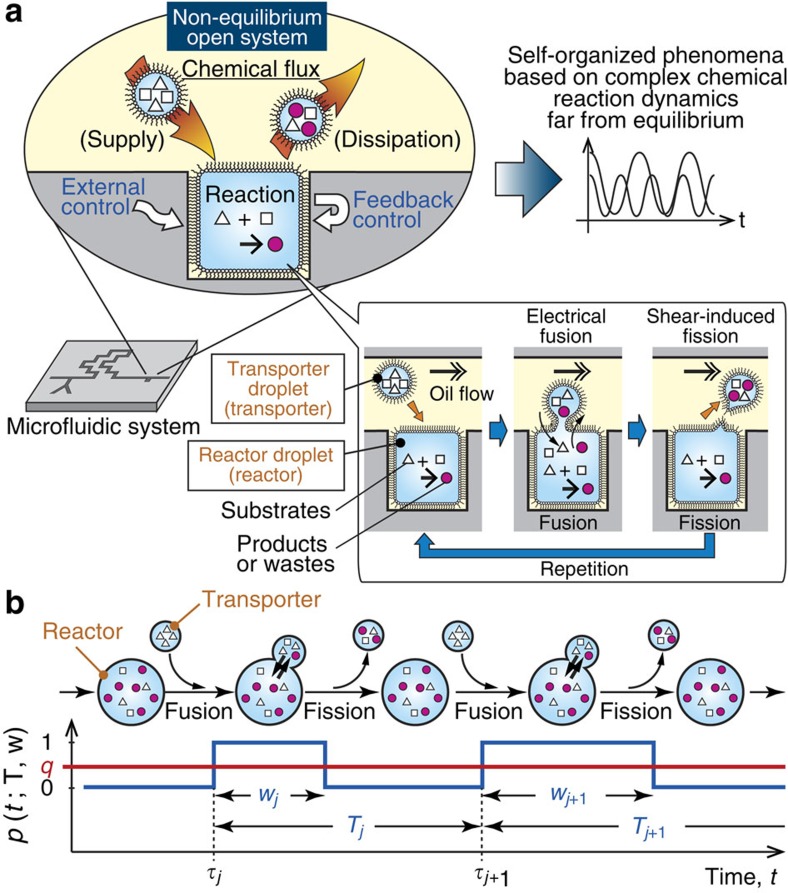
Figure 1. Schematic diagram of chemical reactions far from equilibrium in a droplet open-reactor system controlled by pulse-density modulation.
(a) In the droplet open-reactor system, the supply of substrates and the dissipation of products/wastes into/out of the reaction system are sustained, inducing self-organized phenomena based on complex chemical reaction dynamics far from equilibrium. The chemical reactions in the droplet open-reactor system are dynamically varied based on external control and feedback control. The droplet open-reactor system is based on the repeated fusion and fission of droplets. (b) A fusion–fission process and the pulse-density modulation concept. Tj and wj are the interval and duration of j-th fusion–fission event, respectively. p(t; T, w) is a square pulse-train function used to express a fusion–fission process (T={Tj}; w={wj}). q is the basal strength of the chemical fluxes. τj is the time at which the j-th fusion starts.