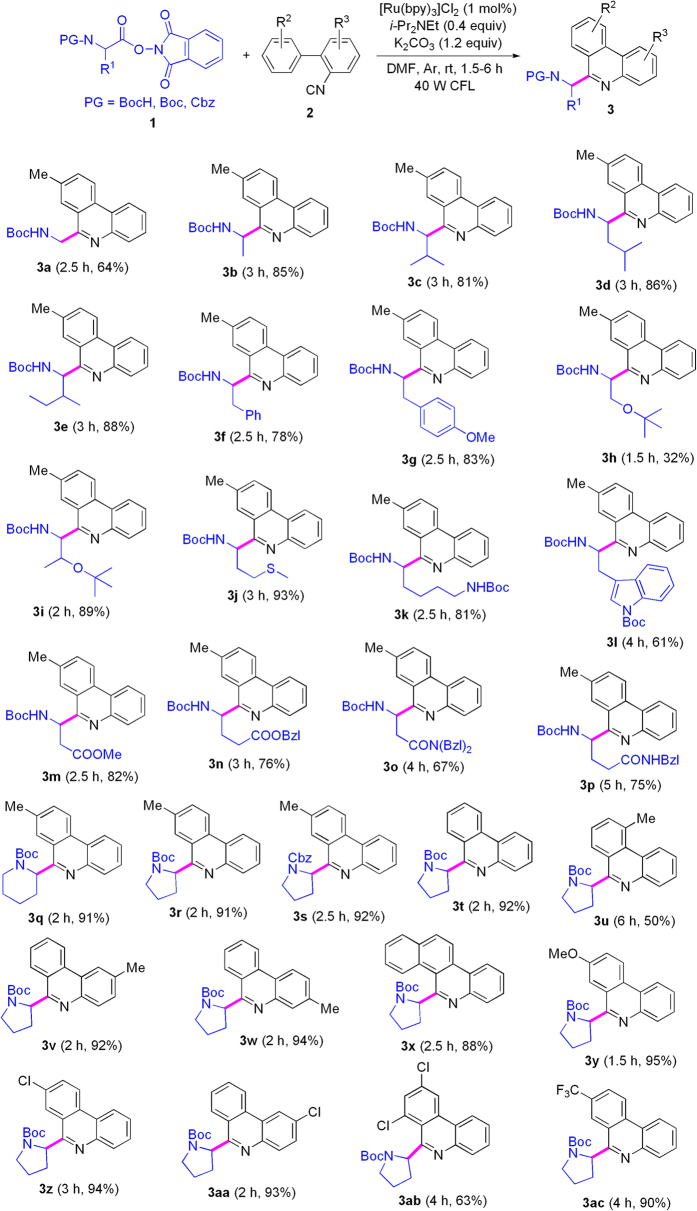
Figure 2. Substrate scope on conjugations of N-protected amino acids and phenanthridines*.
*Reaction conditions: under Ar atmosphere and irradiation of visible light, N-protected amino acid-OPht (1) (0.45 mmol for synthesis of 3a–p; 0.30 mmol for synthesis of the others), substituted 2-isocyanobiphenyl (2) (0.15 mmol), [Ru(bpy)3]Cl2 (1.5 μmol), DIPEA (0.06 mmol), K2CO3 (0.18 mmol), DMF (2.0 mL), temperature (rt, ~25 oC), time (1.5–6 h) in a sealed Schlenk tube. †Isolated yield. Boc = tert-butyloxycarbonyl. Bzl = benzyl. Cbz = benzyloxycarbonyl.