Table 1. Development of a method for installing N-Boc proline residue on 8-methylphenanthridine*.
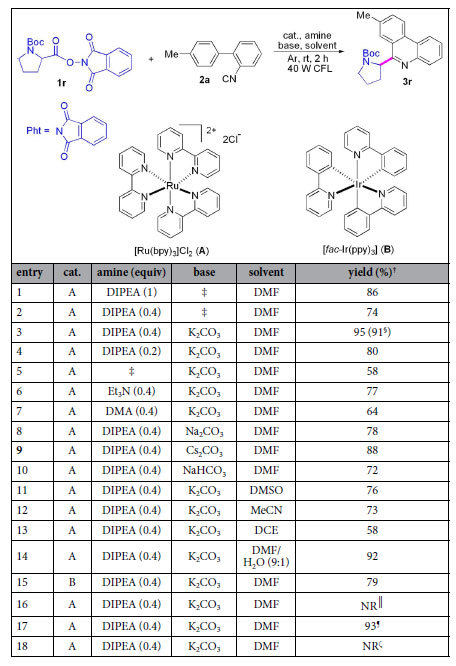
*Reaction conditions: under Ar atmosphere and irradiation of visible light, N-Boc-Pro-OPht (1r) (0.30 mmol), 1-isocyano-(p-phenyl)-benzene (2a) (0.15 mmol), catalyst (1.5 μmol), amine (0.03–0.15 mmol), base (0.18 mmol), solvent (2.0 mL), temperature (rt, ~25 oC), time (2 h) in a sealed Schlenk tube. †Conversion yield by 1H NMR determination using trichloroethylene as the internal standard. ‡No addition of reagent. §Isolated yield. ║Under air. ¶Under irradiation of blue LED. ζNo light. DIPEA = diisopropylethylamine. DCE = 1,2-dichloroethane. DMA = N, N- Dimethylaniline. CFL = compact fluorescent light.
