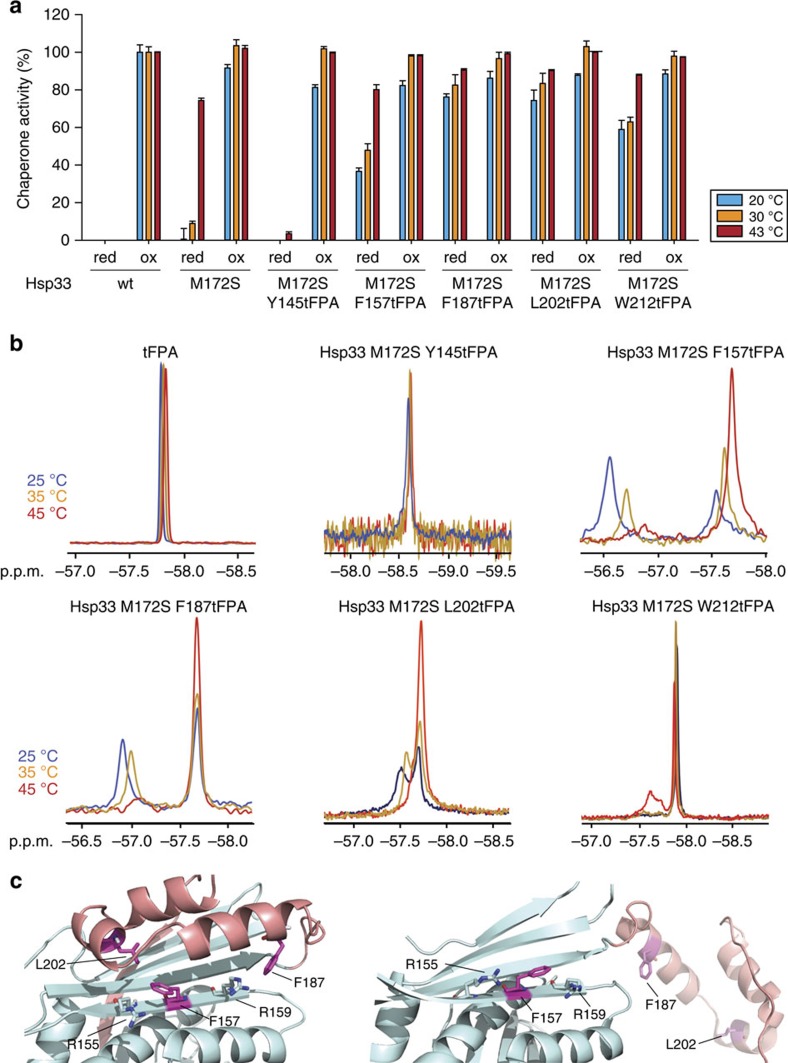
Figure 2. Monitoring conformational rearrangements in purified Hsp33M172-tFPA variants using 19F NMR.
(a) Chaperone activity of reduced, zinc-reconstituted or HOCl-activated wild-type Hsp33, Hsp33M172S or Hsp33M172S-tFPA variants. Chaperone activity was measured by testing the influence of a four-fold molar excess of Hsp33 on the aggregation of chemically unfolded CS at either 20 °C (blue bars) or 30 °C (orange bars) or on thermally unfolded CS at 43 °C (red bars). Chaperone activity of 0% is defined as the light-scattering signal 4 min after addition of CS in the absence of chaperones. Activity of 100% corresponds to the light-scattering signal of CS in the presence of a four-fold molar excess of wild-type Hsp33 that had been activated for 2 min in 200 μM HOCl at 30 °C. All experiments were conducted at least 3–5 times and the s.e.m. is shown. (b) Temperature dependence of the 19F NMR signal in select Hsp33M172S-tFPA mutants. 19F NMR spectra of tFPA alone or the indicated mutant variants were recorded at either 25 °C (blue), 35 °C (orange) or 45 °C (red). (c) N-terminal linker-docking surface (cyan) and the metastable linker region (pink) of E. coli Hsp33 in the inactive, closed state (left) (I-TASSER model) and in the activated, open state (right) (PDB 1HW7). The close proximity of F157 and F187 (and L202) to Arg155 and Arg159 in the closed state of Hsp33 is likely responsible for the distinctive down-field chemical shift change observed in the mutant variants under inactivating conditions.