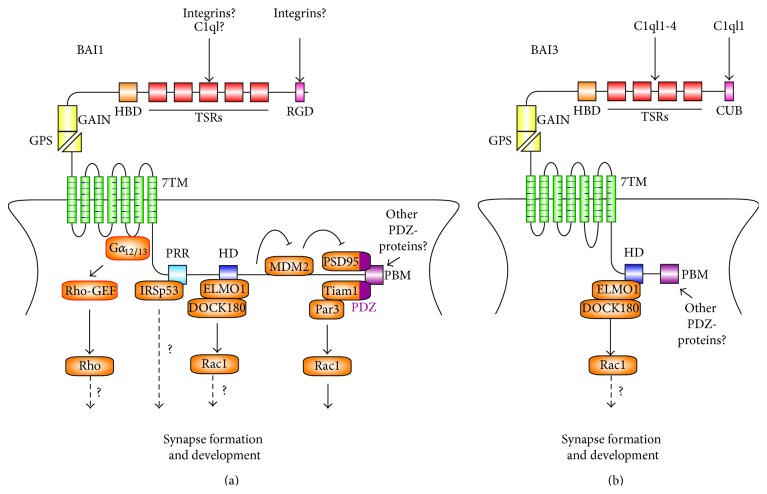
Figure 2.
Synaptic binding partners and signaling pathways of BAI adhesion-GPCRs. (a) Synaptic interactions of BAI1. On the N-terminal segment of BAI1, the TSRs and the RGD motif are predicted to bind integrins. The TSRs also putatively bind complement C1ql factors, although the function of this interaction is unclear. BAI1 activates the RhoA pathway by coupling with Gα 12/13, although this has only been shown in cultured HEK293T cells and requires confirmation in neurons (red outline). The C-terminal region of BAI1 binds to IRSp53 via its proline-rich region (PRR), but the function of this interaction needs to be further explored. BAI1 also interacts with the Rac1 activator modules ELMO1/DOCK180 (via the α-helical RKR motif (HD)) and Tiam1/Par3 (via the PDZ-binding motif (PBM)). However, only the Tiam1/Par3 interaction is required for BAI1's effects on dendritic spine formation and excitatory synaptogenesis. In addition, BAI binds to the ubiquitin E3 ligase MDM2 and suppresses its polyubiquitination activity on PSD95, stabilizing PSD95 expression levels. (b) Synaptic interactions of BAI3. The TSRs and the CUB domain of BAI3 have been shown to bind complement C1ql factors C1ql3 and C1ql1, respectively. In cerebellar development, the C1ql1-BAI3 interaction helps establish proper synaptic connectivity in Purkinje cells and maintain a single-winner climbing fiber. The α-helical RKR motif (HD) of BAI3 also interacts with ELMO1/DOCK180 to regulate dendritogenesis, but the role of this interaction in synaptogenesis remains to be determined.