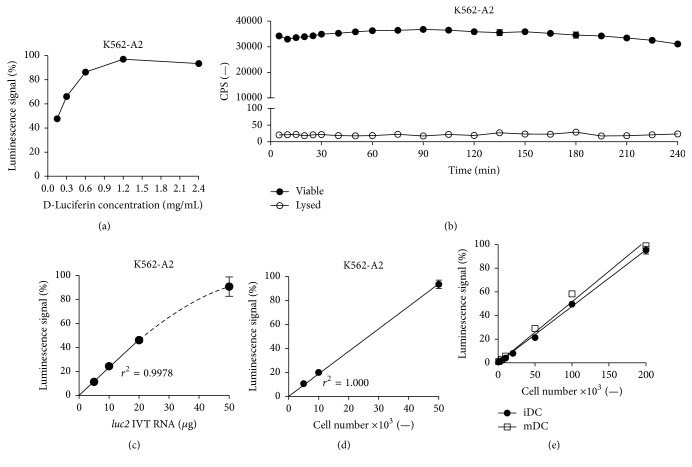
Figure 2.
Optimization of the assay parameters enhances and prolongs luciferase signals whilst minimizing background reading and reveals a strict luminescence to cell number correlation. (a) Optimal D-luciferin substrate concentration. 1 × 106 K562-A2 cells were transfected with 20 μg luc2 IVT RNA. After 24 h, luminescence of 5 × 104 cells per well was measured following addition of D-luciferin in different concentrations. (b) Stable bioluminescence upon D-luciferin substrate addition and immediate abolition of signals following total cell lysis. 2.5 × 106 K562-A2 cells were transfected with 50 μg luc2 IVT RNA. 24 h after transfection, luminescence of 5 × 104 viable or 0.2% Triton X-100 treated cells was repeatedly measured after a single administration of 1.2 mg/mL D-luciferin substrate. Graph represents mean ± SD luminescence (n = 3). (c) Luminescence is dependent on luc2 IVT RNA dose. 1 × 106 K562-A2 cells were transfected with different amounts of luc2 IVT RNA. 24 h after transfection, luminescence of 5 × 104 cells per well was measured. (d) Luminescence is linearly dependent on the number of transfected K562-A2 cells. 1 × 106 K562-A2 cells were transfected with 50 μg luc2 IVT RNA. 24 h after transfection, 1.2 mg/mL D-luciferin was added and luminescence of different amounts of cells was measured. (e) Luminescence is linearly dependent on the number of transfected primary cells. Human iDCs and mDCs were electroporated with 50 μg luc2 IVT RNA. 24 h after transfection, 1.2 mg/mL D-luciferin was added and luminescence of different amount of cells was measured. Cell number and signal intensity correlation (p < 0.0001, r 2 = 0.9984 (iDC) and 0.9923 (mDC)). Graphs (a), (c), (d), and (e) represent mean ± SD luminescence (n = 3) relative to the highest luminescence signal obtained within each experiment.