Fig. 1.
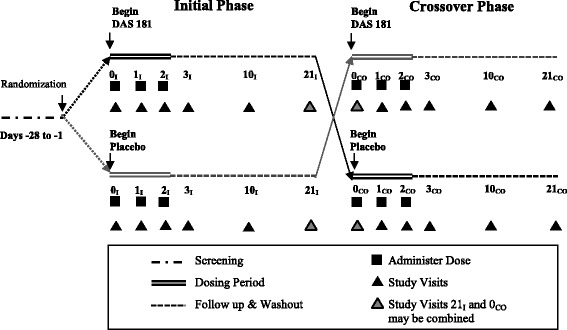
Schematic of study design. Subjects who met eligibility criteria during screening were randomized to receive three consecutive daily doses of either DAS 181 or placebo starting on day 0, the first day of the initial period. Subjects were evaluated at specified time-points for an additional 18 days, then crossed-over to the other treatment group within 6 weeks of completing the initial period. Abbreviations: I = initial; CO = crossover
